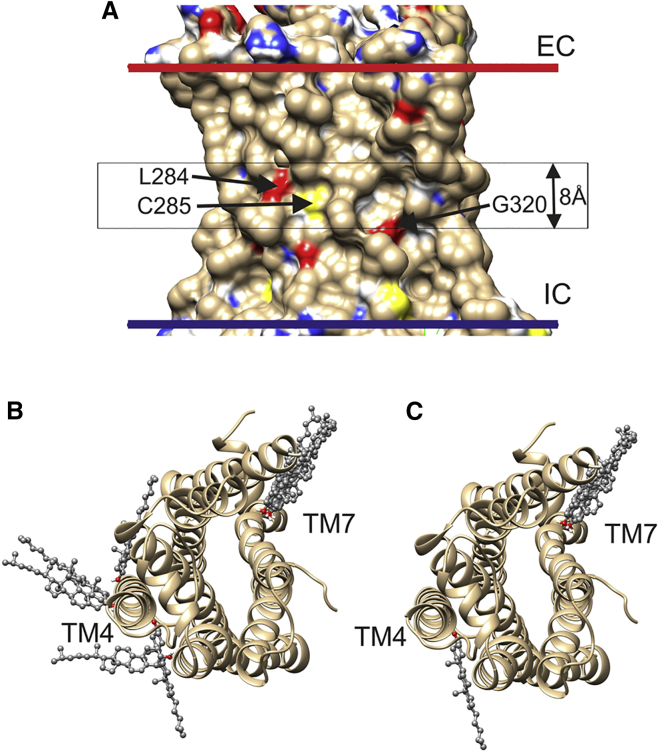
Figure 1.
Docking of cholesterol to the agonist-free β2 adrenergic receptor (PDB: 3D4S). (A) The membrane-spanning surface shows the locations of surface-exposed oxygen (red), nitrogen (blue), and sulfur (yellow) atoms not involved in intramolecular hydrogen bonding. The extracellular (EC) and intracellular (IC) sides of the hydrophobic domain of the membrane surrounding the protein, as given by the OPM database, are shown by red and blue bars, respectively. The central black box shows the position of the 8 Å slab used for docking. The three residues containing surface-exposed, non-hydrogen-bonded O and S atoms located within the box and visible in this view are labeled. (B) The 10 most energetically favorable of the 20 docking poses before selection for hydrogen bonding are shown. The view is from the EC side. (C) The six poses remaining after selection for cholesterol molecules hydrogen bonding to residues not involved in intramolecular hydrogen bonding are shown. These poses involve hydrogen bonding to Ser161 in TM4 and Gly320 in TM7. To see this figure in color, go online.