Abstract
This study aimed to test pharmaceutical compounds targeting astrocytes showing inflammatory dysregulation. The primary rat brain cultures were treated with different batches of serum with or without microglia added to make the cells inflammatory-reactive. Lipopolysaccharide (LPS) and tryptase were used as inflammatory inducers. Expression levels of Toll-like receptor 4 (TLR4), Na+/K+-ATPase, and matrix metalloprotease-13 (MMP-13), as well as actin filament organization, pro-inflammatory cytokines, and intracellular Ca2+ release, were evaluated. LPS combined with tryptase upregulated TLR4 expression, whereas Na+/K+-ATPase expression was downregulated, ATP-evoked Ca2+ transients were increased, actin filaments were reorganized and ring structures instead of stress fibers were observed. Other aims of the study were to prevent astrocytes from becoming inflammatory-reactive and to restore inflammatory dysregulated cellular changes. A combination of the μ-opioid antagonist (−)-naloxone in ultra-low concentrations, the non-addictive μ-opioid agonist (−)-linalool, and the anti-epileptic agent levetiracetam was examined. The results indicated that this drug cocktail prevented the LPS- and tryptase-induced inflammatory dysregulation. The drug cocktail could also restore the LPS- and tryptase-treated cells back to a normal physiological level in terms of the analyzed parameters.
Keywords: Astrocytes, Inflammation, Ca2+ signaling, Actin filament, (−)-Naloxone, (−)-Linalool, Levetiracetam
Highlights
-
•
New combinations of pharmaceutical compounds can resolve and restore disordered cellular inflammatory pathways in network coupled cells.
-
•
Gap junctions coupled cells may be targets of inflammation.
-
•
The new combination has been tested using in vitro networks of astrocytes.
-
•
Clinical consequences of chronic inflammation in one or several organs are increased risks for co-morbidity seen in several diseases.
1. Introduction
Inflammatory cells are important contributors to the pathophysiological response to injury. Penetration through the blood–brain barrier (BBB) is achieved by circulating bone-marrow-derived leukocytes or monocytes, which can transform into macrophages/microglia. These cells secrete cytokines that promote tissue damage (Medzhitov, 2008, Waisman et al., 2015). The BBB consists of capillary endothelial cells containing specialized tight junctions and surrounded by a basement membrane, which is composed of extracellular matrix components, pericytes and astrocytic perivascular endfeet (Abbott et al., 2006). The gram-negative bacterial endotoxin lipopolysaccharide (LPS) induces the conversion of endothelial cells into activated fibroblasts that show a myofibroblast-like protein profile. This process is mediated by the Toll-like receptor 4 (TLR4)/NF-κB pathway (Sarmiento et al., 2014).
Astrocytes are now known to exert either potent pro-inflammatory functions or crucial protective anti-inflammatory functions that are regulated by specific signaling inputs (Hansson, 2010, Zeng et al., 2013, Hansson and Skiöldebrand, 2015). Their processes establish contacts with the BBB and the processes of other astrocytes via gap junctions, thereby forming networks of coupled astrocytes (Cornell-Bell et al., 1990, Blomstrand et al., 1999, Guthrie et al., 1999, Nedergaard et al., 2003). At sites of CNS tissue damage, astrocytes become reactive and form scar borders, which serve as functional barriers and have the potential to release diverse molecules that affect nearby cells. Astrocyte dysfunction can be caused by genetic polymorphisms or by exposure to molecular signals derived from infections or trauma, which can alter astrocyte inflammatory regulation and lead to detrimental effects (Sofroniew, 2015), including the alteration of astrocyte signaling mechanisms (Hansson, 2010, Hansson, 2015).
LPS as a potent inflammatory activator in astrocytes, has shown to give good results (Forshammar et al., 2011, Block et al., 2012, Block et al., 2013). However, better inflammatory-induced activity is desired as the mechanisms of actions in the restoration processes are desired to be better expressed.
Mast cells are derived from the bone marrow and play important roles in inflammation, immune responses and tissue repair. They circulate in an immature form until they reach the target tissue site (Skaper et al., 2013). Mast cells are rich in proteases, tryptases and chymases, as well as cytokines such as interleukins, TGFβ and tumor necrosis factor-α (TNF-α). They contact only blood vessels that are ensheathed by astroglial processes, and they can alter the BBB permeability and pass into the CNS (Kahli et al., 2007, Dong et al., 2014). They can upregulate purinergic receptors on astrocytes (Dong et al., 2014) and activate the G protein-coupled protease-activated receptor (PAR-2) in astrocytes by releasing tryptase (Zeng et al., 2013).
Matrix metalloproteinases (MMPs or matrixins) activate signal transduction pathways that control cytokine biosynthesis and barrier immunity. MMP-13, which is produced by both neurons and astrocytes, is upregulated in response to inflammation in the brain, and it remodels the extracellular matrix and degrades substrates as part of the neuroinflammatory response (Cuadrado et al., 2009).
The first aim of the present study was to test different substances of relevance to get astrocytes more inflammatory reactive compared to LPS-induced inflammatory-reactivity. The astrocyte cultures were treated with different batches of serum with or without microglia. Furthermore, LPS and tryptase were applied in combination. Cellular changes were analyzed according to several parameters, such as expression of TLR4, Na+/K+-ATPase, and MMP-13, as well as actin filament organization, pro-inflammatory cytokine levels, and intracellular Ca2+ release. The second aims were to 1) prevent astrocytes from becoming inflammatory-reactive and 2) restore cellular changes and disturbances, which already were inflammatory-reactive back to physiological levels. We have earlier shown that a combination of a μ-opioid receptor antagonist in ultra-low concentrations, naloxone, a μ-opioid agonist, endomorphin-1, and an agent attenuating IL-1β release, levetiracetam, can restore cellular parameters induced by inflammation. The responses have been tested in astrocytes (Block et al., 2013) and in post-surgical neuropathic pain patients in vivo (Block et al., 2015). We wanted now to replace endomorphin-1/morphine with the non-addictive μ-opioid agonist (−)-linalool and evaluate if this substance had similar restorative effects as endomorhin-1.
2. Materials and methods
The studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 or the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, or the European Communities Council Directive of 24 November 1986 (86/609/EEC).
2.1. Primary astrocyte cultures and treatments
Primary cortical astrocytes, from Sprague-Dawley rats at embryonic day 19, were purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA) and prepared according to the manufacturer's instructions with some modifications. Briefly, one vial containing 1 × 106 viable cells were plated at a seeding density of 1 × 104 cells per cm2 on uncoated glass coverslips (no. 1, 20 mm in diameter) (Bergman Labora, Stockholm, Sweden) and placed in 12-well plates. The medium was replaced twice per week. The astrocytes were used after 16–17 days in culture.
2.2. Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.3. Inflammatory-reactive substances added to astrocyte cultures
Cultures were treated with two different batches of serum: 15% fetal bovine serum from Invitrogen, called astroglia serum, or 15% fetal bovine serum (Biochem AG, Berlin, Germany) called microglia serum, as it was used to increase the amount of microglia (Persson et al., 2005). Some cultures were shaken to promote growth of microglia. Exogenous microglia were added to the astrocyte cultures.
LPS (10 ng/ml) (Forshammar et al., 2011), which was used to promote inflammatory reactivity, was added 24 h before the experiments. To further increase the inflammatory reactivity, tryptase (10 ng/ml) (Zeng et al., 2013) was added together with LPS 24 h before the experiments.
2.4. Immunocytochemistry
Immunocytochemistry was done according to Block et al. (2013). Glial fibrillary acidic protein (GFAP) (Dako, Glostrup, Denmark) and a mouse monoclonal antibody against OX42 (Serotec, Oxford, UK) were used. The cells were viewed using a Nikon Eclipse 80i microscope. Pictures were taken with a Hamamatsu C5810 color-intensified 3CCD camera.
2.5. Viability assay
A LIVE/DEAD viability assay kit (Invitrogen Molecular Probes) for mammalian cells was used according to Forshammar et al. (2011). viewed using a Nikon Eclipse 80i microscope. Pictures were taken with a Hamamatsu C5810 color-intensified 3CCD.
2.6. Calcium imaging
Calcium imaging was done as earlier described (Block et al., 2013). The total areas under the curve (AUC), which reflects the amount of Ca2+ released (Berridge, 2007), were analyzed. The amplitude was expressed as the maximum increase of the 340/380 ratio. The area under the Ca2+ peaks (AUC) was calculated in Origin (Microcal Software Inc., Northampton, MA, USA). Forty cells were used for each experimental set-up and were taken from four different coverslips and from two different seeding times.
2.7. SDS-PAGE and western blot
SDS-page and western blot were done as earlier described (Block et al., 2013).
2.8. Actin visualization
The astrocyte cytoskeleton was stained using Alexa™488-conjugated phalloidin (Invitrogen) (Block et al., 2013).
2.9. Actin assay
Actin quantitation was performed as recommended by the F-actin/G-actin In Vivo Assay Biochem Kit (Cytoskeleton, Inc., Denver, CO, USA) and SDS-page performed as described above, with the exception of the sample preparation. The primary antibody, rabbit polyclonal anti-actin (Cytoskeleton, 1:500), and the secondary antibody, HRP-conjugated donkey anti-rabbit IgG F(ab′)2 fragments (Jackson ImmunoResearch, 1:10,000), were diluted in blocking solution.
2.10. Cytokine release
Rat IL-1β (R&D Systems, Oxon, UK) and TNF-α (Becton Dickinson, NJ, USA) were used to measure the amounts of cytokines via ELISA according to the manufacturer's instructions.
2.11. Protein determination
A protein determination assay was performed in accordance with the manufacturer's instructions using a detergent-compatible (DC) Protein Assay (Bio-Rad, Hercules, CA, USA) and based on the method used by Lowry et al. (1951).
2.12. Statistics
Statistical significance was determined using Student's paired t-test or one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test. The error bars represent the standard error of the mean (SEM).
3. Results
3.1. Activation of astrocytes to become inflammatory-reactive
3.1.1. Different batches of serum and/or addition of microglia
The results show that cultivating the astrocytes used in the present experiments, which is cells bought from a company, in different serum batches or shaking the cultures in different ways to stimulate microglia growth did not increase the inflammatory reactivity as evaluated with the present parameters. This was, however, not the case with our previous astrocytes made by ourselves (Hansson et al., 1984). Furthermore, addition of external microglia to the present astrocyte cultures did not increase the inflammatory reactivity of the astrocytes.
In all further experiments, the cells were cultivated with astroglia serum without the addition of exogenous microglia.
3.1.2. Astrocytes incubated with LPS or LPS and tryptase
Cultures were stained for OX42, a marker for microglial cells, and for GFAP, a marker for astrocytes. Very few or no microglia were found in the control cultures or in cultures treated with LPS for 24 h or with LPS + tryptase for 24 h (Fig. 1). There were small differences among the groups in the viability of the cells visualized with a LIVE/DEAD kit (Fig. 2).
Fig. 1.
Culture stained for OX42, a marker for microglial cells (green), and for GFAP, a marker for astrocytes (red). A. Very few microglia were found in the control cultures. B. Similarly, very few microglia were found after treatment with LPS for 24 h or with C. LPS + tryptase for 24 h. Scale bar = 50 μm. Representative images are presented.
Fig. 2.
Cell viability was visualized with Calcein AM (green) using a LIVE/DEAD kit and showed very few dead cells, shown with red nuclei in figure (A). The viability did not change after incubation with LPS (B) or with LPS and tryptase (C). Scale bar = 50 μm. Representative images are presented.
Astrocyte cultures were incubated with LPS for 24 h. They showed an increase in TLR4 expression (P < 0.05) (Fig. 3), a decrease in Na+/K+-ATPase expression (P < 0.05) (Fig. 4), and no change in MMP-13 expression (Fig. 5). Furthermore, the cells were stimulated with ATP (10−4 M), which increased the ATP-evoked Ca2+ transients, AUC, (P < 0.001) (Fig. 6). The astrocytes were stained with an Alexa488-conjugated phalloidin probe. The untreated cultures were dominated by F-actin organized in stress fibers (control). The cultures incubated with LPS showed a more diffuse organization of actin filaments, and the ring structures were more pronounced (Fig. 7). They also showed morphologic changes, including the retraction of cell bodies and changes in the stress fiber distribution. Therefore, the F/G actin ratio was calculated, and it was increased in the cells treated with LPS (P < 0.01) (Fig. 9).
Fig. 3.
TLR4 expression. Astrocytes were incubated with LPS (10 ng/ml) for 24 h or with LPS (10 ng/ml) and tryptase (100 ng/ml) for 24 h. The expression of TLR4 was increased compared with the control. The level of significance was analyzed using a one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05. n = 5.
Fig. 4.
Na+/K+-ATPase expression. Astrocytes were incubated with LPS (10 ng/ml) for 24 h or with LPS (10 ng/ml) and tryptase (100 ng/ml) for 24 h. The Na+/K+-ATPase expression was decreased compared with the control. The level of significance was analyzed using a one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05. **P < 0.01. n = 5.
Fig. 5.
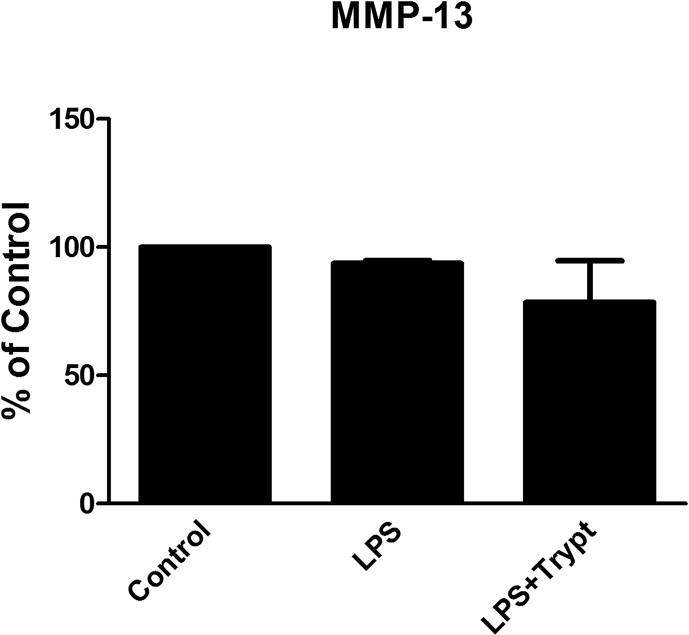
MMP-13 expression. Astrocytes were incubated with LPS (10 ng/ml) for 24 h or with LPS (10 ng/ml) and tryptase (100 ng/ml) for 24 h. The MMP-13 expression was not significantly changed. The level of significance was analyzed using a one-way ANOVA followed by Dunnett's multiple comparisons test. n = 5.
Fig. 6.
ATP-evoked Ca2+transients. All astrocytes were stimulated in a Ca2+ imaging system. The cells were incubated with a Ca2+-sensitive fluorophore probe (Fura-2/AM). ATP (10−4 M) elicited Ca2+ responses in the astrocytes and was used as the control. The area under the Ca2+ peak (AUC) was calculated for each Ca2+ transient. The cells incubated with LPS (10 ng/ml) for 24 h showed increased Ca2+ signaling, as did the cells incubated with LPS (10 ng/ml) and tryptase (100 ng/ml) for 24 h. The level of significance was analyzed using a one-way ANOVA followed by Dunnett's multiple comparisons test. **P < 0.01, ***P < 0.001. n = 5.
Fig. 7.
Actin filaments. Astrocytes were stained with Alexa488-conjugated phalloidin. A. The untreated cells were dominated by F-actin organized in stress fibers (control). B. The cells incubated with LPS (10 ng/ml) for 24 h showed a more diffuse organization of actin filaments, and the ring structures were more pronounced. C. The cultures incubated with LPS (10 ng/ml) and tryptase (100 ng/ml) for 24 h showed an even more diffuse organization than LPS-treated cells. Scale bar = 50 μm. Representative images are presented.
Fig. 9.
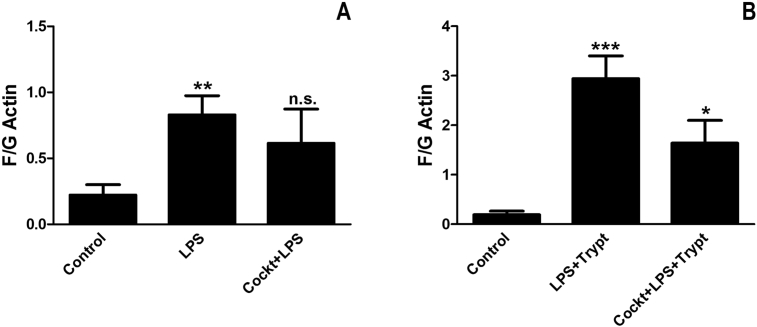
Actin filaments. Astrocytes were incubated with a cocktail of naloxone (10−12 M), -(−)linalool (10−6 M), and levetiracetam (10−4 M) for 30 min. Then, the incubation continued with the cocktail and LPS (10 ng/ml) (A), or the cocktail and LPS + tryptase (100 ng/ml) for 24 h (B). The F/G actin ratio was measured. The result showed that the ratio was increased in the cells treated with LPS and LPS + tryptase, and decreased when the cells were pretreated with the cocktail. The level of significance was analyzed using one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. = non-significant. n = 4.
Astrocyte cultures were incubated with LPS + tryptase for 24 h. They showed an increase in TLR4 expression (P < 0.05) (Fig. 3), a decrease in Na+/K+-ATPase expression (P < 0.01) (Fig. 4), and no change in MMP-13 expression (Fig. 5). Furthermore, when the cells were stimulated with ATP (10−4 M), the ATP-evoked Ca2+ transients increased, AUC, (P < 0.01) (Fig. 6). The cells changed their morphology in comparison to astrocytes incubated with only LPS (Fig. 7), and the F/G actin ratio was increased in the cells treated with LPS and tryptase (P < 0.001) (Fig. 9).
None of the above combinations showed release of IL-1β or TNF-α.
3.2. Prevent LPS- and tryptase-induced biochemical changes in astrocytes with the combination of (−)-naloxone, (−)-linalool and levetiracetam
Astrocytes were incubated with a cocktail of naloxone (10−12 M), (−)-linalool (10−6 M), and levetiracetam (10−4 M) for 30 min. The incubation continued with the cocktail and LPS or with the cocktail and LPS + tryptase for 24 h. The TLR4 expression was decreased when incubated with the cocktail, which showed the same expression level as the control cells. The cocktail prevented the cells to become inflammatory-reactive. The Na+/K+-ATPase expression was increased, whereas MMP-13 expression did not change (Fig. 8). The F/G actin ratio was calculated and was increased in the cells treated with LPS (P < 0.01) or LPS + tryptase (P < 0.001) but was decreased in the presence of the cocktail (Fig. 9). The actin filaments were prevented from reorganization.
Fig. 8.
Prevent LPS- and tryptase-induced biochemical changes in astrocytes with the combination of (−)-naloxone, (−)-linalool and levetiracetam. Astrocytes were incubated with a cocktail of naloxone (10−12 M), (−)-linalool (10−6 M), and levetiracetam (10−4 M) for 30 min. Then, the incubation continued with the cocktail and LPS (10 ng/ml), + tryptase (100 ng/ml) for 24 h. The expression levels of TLR4, Na+/K+-ATPase and MMP-13 were studied using western blot analysis. The level of significance was analyzed using one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05, ***P < 0.001, n.s. = non-significant. n = 4.
3.3. Restore LPS- and tryptase-induced biochemical changes in astrocytes with the combination of (−)-naloxone, (−)-linalool and levetiracetam
Astrocytes were incubated with LPS + tryptase for 24 h. Then, the incubation continued with LPS + tryptase and the cocktail of naloxone (10−12 M), (−)-linalool (10−6 M), and levetiracetam (10−4 M) for an additional 24 h. The expression levels of TLR4, Na+/K+-ATPase, and MMP-13 were then analyzed (Fig. 10). The cocktail restored the increased expression of TLR4 to the control level. A similar effect was seen for Na+/K+-ATPase, but MMP-13 expression did not change. The F/G actin ratio was calculated and was increased in the cells treated with LPS (P < 0.05) and LPS + tryptase (P < 0.001) compared to their respective controls. The cocktail decreased the ratio back to the control level (Fig. 11).
Fig. 10.
Restore LPS- and tryptase-induced biochemical changes in astrocytes with the combination of (−)-naloxone, (−)-linalool and levetiracetam. Astrocytes were incubated with LPS (10 ng/ml) + tryptase (100 ng/ml) for 24 h. Then, the cells were incubated with LPS + tryptase and the cocktail of naloxone (10−12 M), (−)-linalool (10−6 M), and levetiracetam (10−4 M) for an additional 24 h. The expression levels of TLR4, Na+/K+-ATPase and MMP-13 were studied using western blot analysis. The level of significance was analyzed using one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05, ***P < 0.001, n.s. = non-significant. n = 4.
Fig. 11.
Actin filaments. Astrocytes were incubated with LPS (10 ng/ml) (A) or LPS + tryptase (100 ng/ml) (B) for 24 h. Then, the incubation continued with LPS and the cocktail of naloxone (10−12 M), (−)-linalool (10−6 M), and levetiracetam (10−4 M) for an additional 24 h (A), or with LPS + tryptase and the cocktail for an additional 24 h (B). The F/G actin ratio was measured. The ratio was increased in the cells treated with LPS and tryptase but decreased when the cells were stimulated with the cocktail. The level of significance was analyzed using one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05, ***P < 0.001, n.s. = non-significant. n = 4.
3.3.1. Glutamate-evoked Ca2+ responses were increased with LPS and attenuated by the combination of naloxone, (−)-linalool and levetiracetam
Glutamate (10−4 M) was used as a stimulator in the Ca2+ imaging experiments, in which all cells responded (n = 40). The AUC was calculated for each condition (Fig. 12).
Fig. 12.
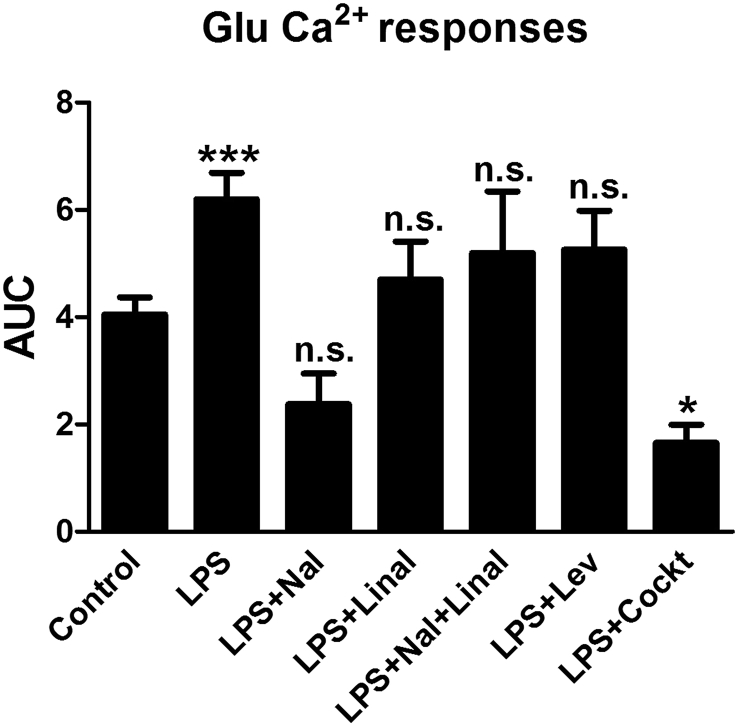
Glutamate-evoked Ca2+transients. All astrocytes were stimulated in a Ca2+ imaging system. The cells were incubated with a Ca2+-sensitive fluorophore (Fura-2/AM). Glutamate (10−4 M) elicited Ca2+ responses in the astrocytes and was used as a control. The area under the Ca2+ peak (AUC) was calculated for each Ca2+ transient. The cells incubated with LPS (10 ng/ml) for 24 h showed increased Ca2+ signaling. The cells incubated with LPS (10 ng/ml) for 24 h and then (−)-naloxone (10−12 M), (−)-linalool (10−6 M), levetiracetam (10−4 M), or a combination of the three substances was applied 3.5 min before glutamate stimulation. (−)-linalool alone, (−)-naloxone alone or together with (−)-linalool, or levetiracetam alone did not attenuate the glutamate-upregulated Ca2+ transients. The combination of (−)-naloxone, (−)-linalool, and levetiracetam (cocktail) attenuated the glutamate signaling. The level of significance was analyzed using a one-way ANOVA followed by Dunnett's multiple comparisons test. *P < 0.05, ***P < 0.001, n.s. = non-significant. n = 5.
Astrocytes incubated with LPS for 24 h showed an increase in glutamate-evoked Ca2+ signaling compared with controls (glutamate stimulation alone). The AUC was increased (P < 0.001). All cells responded, n = 40 (Fig. 12). The cells were incubated with LPS (10 ng/ml) for 24 h, and then (−)-naloxone (10−12 M), (−)-linalool (10−6 M), and levetiracetam (10−4 M) were applied 3.5 min before glutamate stimulation. Notably, the glutamate-upregulated Ca2+ increase was not attenuated by (−)-linalool alone, (−)-naloxone alone or combined with (−)-linalool, or levetiracetam alone. However, the combination of (−)-naloxone, (−)-linalool, and levetiracetam (cocktail) attenuated the glutamate signaling (P < 0.05), and all cells responded (Fig. 12). The present results have to be compared with the results in Block et al. (2013), where the cocktail included endomorphin-1 instead of (−)-linalool. The present results show that endomorphin-1 can be replaced by the non-addictive μ-agonist (−)-linalool.
4. Discussion
Inflammation in the CNS differs from inflammation elsewhere in the body because of the sensitivity and isolation of the CNS. Microglia produce anti-inflammatory and neurotrophic factors under physiological conditions and produce pro-inflammatory mediators in response to infection or tissue damage (Streit, 2002). They act on astrocytes that in turn amplify the inflammatory reaction (Saijo et al., 2009). Therefore, microglia and astrocytes are of particular interest because both cell types can initiate and amplify inflammation and thereby release neurotoxic compounds. Therefore, an in vitro cell model system of inflammation is desirable for studying potential treatments, which is even better than the one used by us in earlier experiments (Block et al., 2013).
The astrocyte cultures were investigated using different cultivation parameters. Two different fetal calf sera were used to promote microglial growth. Furthermore, the cultures were shaken to recruit more microglia. Microglia from external cultures were also added. We hypothesized that the number of microglia and/or the reactivity of these cells could make the astrocytes inflammatory-reactive (DeLeo et al., 2004, Milligan and Watkins, 2009). Our results show that astrocytes and microglia are not capable of initiating inflammation by themselves, but microglia do appear to be at least partly responsible for the changes induced in known biomarkers, with a stronger induction of TLR4 and reduced expression of Na+/K+-ATPase, but without the release of pro-inflammatory cytokines.
Previous studies have produced inflammatory-reactive astrocytes with LPS or IL-1β (Forshammar et al., 2011, Lundborg et al., 2011, Block et al., 2012). Mast cells are active in vivo during inflammation and release serine proteases such as tryptase (Medzhitov, 2008). Therefore, we expanded our inflammatory potential combining LPS with tryptase. The tryptase-activated receptor 2 (PAR-2) is well known to be widely expressed on astrocytes and to play a major role in inflammation (Zeng et al., 2013).
TLRs, which recognize structural motifs that are characteristic of pathogens and which are expressed by innate immune cells (Janeway and Medzhitov, 2002), are gaining increasing recognition for their roles in several inflammatory diseases. LPS has been shown to be an agonist for TLR4 (Qureshi et al., 1999), which is thought to be largely responsible for LPS-related signaling (Kielian, 2006). It has been proposed that TLRs drive inflammation that gives rise to detectable symptoms (O'Neill, 2003, Krasowska-Zoladek et al., 2007). Astrocytes express TLR4, which is further induced after incubation with LPS (Forshammar et al., 2011) or with the combination of LPS and tryptase.
Three Na+ ions are required for the uptake of one glutamate, and this transport is dependent on the intra- and extracellular concentrations of Na+ (Longuemare et al., 1999). The intracellular concentration of Na+ is maintained by Na+/K+-ATPase and is dependent on ATP (Longuemare et al., 1999). The present study showed a decrease in Na+/K+-ATPase expression, which was further decreased after the combined treatment with LPS and tryptase.
No changes were observed in the expression of the latent proenzyme form of MMP-13, and no active form of MMP-13 was detected.
In conditions that lead to chronic neuroinflammation, Ca2+ signaling in astrocyte networks is overactivated (Hansson, 2010, Hansson, 2015, Forshammar et al., 2011). Regulation of Ca2+ dynamics by transmitters and soluble factors is a possible mechanism by which the astrocyte networks detect changes in the CNS microenvironment and regulate brain activity, such as inflammatory processes. LPS changed the ATP-evoked Ca2+ transients with increased intracellular Ca2+ release. Intercellular Ca2+ communication takes place through gap junctions, and the parallel system for intercellular Ca2+ communication between astrocytes occurs via extracellular communication through the diffusion of ATP (Fields and Burnstock, 2006). ATP then binds to purinoceptors, specifically P2Y-receptors, which leads to an overstimulation of the Gq protein, and subsequent elevations of intracellular Ca2+ waves are observed (Blomstrand et al., 1999).
Cells treated with LPS or LPS + tryptase displayed reorganization of their stress fibers and induced formation of ring structures that resulted in an increased F/G actin ratio, which was also confirmed using phalloidin staining. Reorganization of F-actin into different contractile structures such as apical rings, vacuoles, or stress fiber-like cables that are more or less diffuse was observed in our previous study (Hansson, 2015), as well as in epithelial cells (Ivanov et al., 2010), pulmonary monocytes (Du et al., 2012), and vascular smooth muscle cells (Kim et al., 2008).
A cocktail of naloxone, (−)-linalool and levetiracetam was used for two purposes. First, to prevent network-coupled astrocytes from becoming inflammatory-reactive. Second, to restore already inflammatory-reactive networks.
Naloxone, a non-selective μ-opioid antagonist, and a (−)-isomer, normally blocks the Gi/Go protein associated with the μ-opioid receptor. However, in ultra-low concentrations, naloxone blocks the Gs protein instead (Block et al., 2012). Chronic morphine administration decreases Gi/Go protein coupling and increases coupling to the Gs protein. This phenomenon has been observed in cultured dorsal root ganglion neurons (Crain and Shen, 1995), rat brain organotypic cultures (Wang and Burns, 2009), in vivo in rats (Wang et al., 2005), and in patients treated for low back pain (Block et al., 2015). Ultra-low concentrations of naloxone can block the Gs protein, and morphine or another μ-opioid receptor agonist can thereby stimulate the Gi/Go protein (Block et al., 2013, Hansson, 2015). However, it is worth knowing that another form of naloxone exists. The inactive (+)-isomer of naloxone, which has no antagonistic effects on opioid receptors, has been shown to be an antagonist of TLR4 and to block the downstream signaling leading to the production of nitric oxide (NO), TNF-α and reactive oxygen species (ROS) (Wang et al., 2015).
An important aim with the present study was to replace endomorphin-1/morphine with a non-addictive μ-opioid agonist (Block et al., 2013). Linalool, (3,7-dimethyl-1,6-octadien-3-ol), a monoterpene, along with its naturally occurring enantiomer (−)-linalool, possesses anti-inflammatory activity, as well as anti-hyperalgesic and anti-nociceptive effects, which are reversed by naloxone and the unselective muscarinic receptor antagonist atropine (Peana et al., 2003, Peana et al., 2006). Furthermore, linalool attenuated the LPS-induced production of TNF-α and IL-6 (Huo et al., 2013). The present results show that (−)-linalool has the same qualities as the previously used μ-opioid receptor agonists.
Levetiracetam has showed to be an effective anti-epileptic drug, and it has also been proved to restore disturbed gap junction coupling in inflammation-reactive astrocyte cultures (Stienen et al., 2010) by increasing connexin 43 expression, the predominant gap junction protein, and to decrease the enhanced production of IL-1β level (Haghikia et al., 2008). The cellular functions of astrocytes in an inflammatory state were virtually restored with the combination of linalool, naloxone and levetiracetam to their normal non-inflammatory states. The present results were comparable with the results by Block et al. (2013), where endomorphin-1, naloxone and levetiracetam were used.
The present results show that the cocktail of naloxone, linalool and levetiracetam has effects promoting the prevention of and recovery from inflammatory reactivity in astrocytes. The three substances in the cocktail affect the cells in different ways. One substance alone has no chance. We previously identified the parameters of the mechanisms underlying or contributing to neuroinflammation (Block et al., 2013, Hansson, 2015). TLR4 is attacked by LPS, and changes in Na+/K+-ATPase seem to be important, as well as Ca2+ signaling in the astrocyte networks, cytokine release and actin filament reorganization. Most parameters were restored to normal physiological levels by the cocktail. The next challenge was to determine whether the astrocyte networks could be restored once they had become inflammatory-reactive upon treatment with LPS + tryptase. Even in this condition, most of the parameters were restored to normal physiological levels by the cocktail.
Glutamate-evoked intracellular Ca2+ release was studied with a Ca2+ imaging system. Glutamate stimulates the astrocytes to release Ca2+, which is further increased with LPS. The single substances in the cocktail released Ca2+ at the same level as glutamate but the challenge was to further decrease the intracellular evoked Ca2+ release. Glutamate is a transmitter with inflammatory properties (Hansson, 1994, Hansson et al., 1994, Milligan and Watkins, 2009). Therefore we reasoned that a further decrease might be of importance. These results were obtained with endomorphin-1 in the cocktail (Block et al., 2013) and with (−)-linalool in the cocktail in the present study.
The next questions and challenges are, of course, whether the cocktail has anti-inflammatory or restorative effects in humans. We have used an ultra-low dose of intrathecal naloxone in addition to intrathecal morphine infusion in patients with severe, persistent pain, which improved the perceived quality of sleep and where conventional pain therapies are insufficient. We were not able to show any statistically significant effects of naloxone on pain relief due to the short treatment time and a small sample size; however, some of the patients in the group reported pain relief (Block et al., 2015, Block, 2016). The next step is to evaluate the present pharmaceutical combination in patients with chronic inflammation and longterm pain for longer time than previously done. Substantial progress has been made in understanding how chronic systemic low-grade inflammation influences the physiology of several diseases such as neuroinflammation, neurodegenerative diseases, osteoarthritis, metabolic, cardiac inflammation and autoimmune diseases, but not why it fails to be resolved (Lécuyer et al., 2016). The physiological nature of inflammation has been gaining ground as a key contributor and search for inflammatory markers has increased. Inflammation requires contribution from other tissue cells such as gap junction coupled cells (Hansson and Skiöldebrand, 2015).
Conclusion: Inflammatory reactivity in astrocytes was achieved with a combination of LPS and tryptase. The pharmaceutical compounds, (−)-linalool, naloxone in ultra-low concentrations, and the anti-epileptic agent levetiracetam, which target dysregulated cellular mechanisms in astrocytes, showed the ability to both prevent and restore inflammatory reactivity. This combination of drugs represents an interesting and new way to influence and restore cellular parameters changed due to inflammation.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Edit Jacobson's Foundation, the Sahlgrenska University Hospital (LUA/ALF GBG-11587) in Gothenburg, Sweden, and AFA Insurance, Stockholm, Sweden (DNr 140329), for financial support.
References
- Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium oscillations. Biochem. Soc. Symp. 2007;74:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- Block L., Forshammar J., Westerlund A., Björklund U., Lundborg C., Biber B., Hansson E. Naloxone in ultralow concentration restores endomorphin-1-evoked Ca2+ signalling in lipopolysaccharide pretreated astrocytes. Neuroscience. 2012;205:1–9. doi: 10.1016/j.neuroscience.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Block L., Björklund U., Westerlund A., Jörneberg P., Biber B., Hansson E. A new concept affecting restoration of inflammation-reactive astrocytes. Neuroscience. 2013;250:536–545. doi: 10.1016/j.neuroscience.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Block L., Lundborg C., Bjersing J., Dahm P., Hansson E., Biber B. Ultralow dose of naloxone as an adjuvant to intrathecal morphine infusion improves perceived quality of sleep but fails to alter persistent pain. A randomized, double-blind, controlled study. Clin. J Pain. 2015;31 doi: 10.1097/AJP.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block L. Glial dysfunction and persistent neuropathic postsurgical pain. Scand. J. Pain. 2016;10:74–81. doi: 10.1016/j.sjpain.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Blomstrand F., Khatibi H., Muyderman H., Hansson E., Olsson T., Rönnbäck L. 5-Hydroxytryptamine and glutamate modulate velocity and extent of intercellular calcium signalling in hippocampal astroglial cells in primary cultures. Neuroscience. 1999;88:1241–1253. doi: 10.1016/s0306-4522(98)00351-0. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A.H., Finkbeiner S.M., Cooper M.S., Smith S.J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signalling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Crain S.M., Shen K.-F. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10540–10544. doi: 10.1073/pnas.92.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado E., Rosell A., Borell-Pagès M., Garcìa-Bonilla L., Hernández-Guillamon M., Ortega—Aznar A., Montaner J. Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J. Cereb. Blood Flow. Metab. 2009;29:398–410. doi: 10.1038/jcbfm.2008.130. [DOI] [PubMed] [Google Scholar]
- DeLeo J.A., Tanga F.Y., Tawfik V.L. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Dong H., Zhang X., Qian Y. Mast cells and neuroinflammation. Med. Sci. Monit. Basic Res. 2014;20:200–206. doi: 10.12659/MSMBR.893093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhou J., Zhang J., Yan M., Gong L., Liu X., Chen M., Tao K., Luo N., Liu J. Actin filament reorganization is a key step in lung inflammation induced by systemic inflammatory response syndrome. Am. J. Respir. Cell Mol. Biol. 2012;47:597–603. doi: 10.1165/rcmb.2012-0094OC. [DOI] [PubMed] [Google Scholar]
- Fields R.D., Burnstock G. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshammar J., Block L., Lundborg C., Biber B., Hansson E. Naloxone and ouabain in ultra-low concentrations restore Na+/K+-ATPase and cytoskeleton in lipopolysaccharide-treated astrocytes. J. Biol. Chem. 2011;286:31586–31597. doi: 10.1074/jbc.M111.247767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P.B., Knappenberger J., Segal M., Bennett M.V.L., Charles A.C., Kater S.B. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia A., Ladage K., Hinkerohe D., Vollmar P., Heupel K., Dermietzel R., Faustmann P.M. Implications of anti-inflammatory properties of the anticonvulsant drug levetiracetam in astrocytes. J. Neurosci. Res. 2008;86:1781–1788. doi: 10.1002/jnr.21639. [DOI] [PubMed] [Google Scholar]
- Hansson E., Rönnbäck L., Persson L.I., Lowenthal A., Noppe M., Alling C., Karlsson B. Cellular composition of primary cultures from cerebral cortex, striatum, hippocampus, brainstem and cerebellum. Brain Res. 1984;300:9–18. doi: 10.1016/0006-8993(84)91335-0. [DOI] [PubMed] [Google Scholar]
- Hansson E. Metabotropic glutamate receptor activation induces astroglial swelling. J. Biol. Chem. 1994;269:21955–21961. [PubMed] [Google Scholar]
- Hansson E., Johansson B.B., Westergren I., Rönnbäck L. Glutamate induced swelling of single astroglial cells in primary culture. Neuroscience. 1994;63:1057–1066. doi: 10.1016/0306-4522(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Hansson E. Long-term pain, neuroinflammation and glial activation. Scand. J. Pain. 2010;1:67–72. doi: 10.1016/j.sjpain.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Hansson E. Actin filament reorganization in astrocyte networks is a key functional step in neuroinflammation resulting in persistent pain: novel findings on network restoration. Neurochem. Res. 2015;40:372–379. doi: 10.1007/s11064-014-1363-6. [DOI] [PubMed] [Google Scholar]
- Hansson E., Skiöldebrand E. Coupled cell networks are target cells of inflammation, which can spread between different body organs and develop into systemic chronic inflammation. J. Inflamm. 2015;12:44. doi: 10.1186/s12950-015-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo M.H., Cui X., Xue J., Chi G., Gao R., Deng X., Guan S., Wei J., Soromou L.W., Feng H., Wang D. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013;180:E47–E54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- Ivanov A.I., Parkos C.A., Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am. J. Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Medzhitov R. Innate immune recognition. Ann. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kahli M., Ronda J., Weitraub M., Jain K., Silver R., Silverman A.-J. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.R., Gallant C., Leavis P.C., Gunst S.J., Morgan K.G. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am. J. Physiol. Cell Physiol. 2008;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowska-Zoladek A., Banaszewska M., Kraszpulski M., Konat G.W. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J. Neurosci. Res. 2007;85:205–212. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- Lécuyer M.A., Kebir H., Prat A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim. Biophys. Acta. 2016;1862:472–482. doi: 10.1016/j.bbadis.2015.10.004. Epub 2015 Oct 8. [DOI] [PubMed] [Google Scholar]
- Longuemare M.C., Rose C.R., Farrell K., Ransom B.R., Waxman S.G., Swanson R.A. K+-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+ Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lundborg C., Westerlund A., Björklund U., Biber B., Hansson E. Ifenprodil restores GDNF-evoked Ca2+ signaling and Na+/K+-ATPase expression in inflammation-pretreated astrocytes. J. Neurochem. 2011;119:686–696. doi: 10.1111/j.1471-4159.2011.07465.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Milligan E.D., Watkins L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M., Ransom B., Goldman S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- O'Neill L.A. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr. Opin. Pharmacol. 2003;3:396–403. doi: 10.1016/s1471-4892(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Peana A.T., D'Aquila P.S., Chessa M.L., Moretti M.D., Serra G., Pippia P. (−)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003;460:37–41. doi: 10.1016/s0014-2999(02)02856-x. [DOI] [PubMed] [Google Scholar]
- Peana A.T.1, Marzocco S., Popolo A., Pinto A. (−)-Linalool inhibits in vitro NO formation: probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 2006;78:719–723. doi: 10.1016/j.lfs.2005.05.065. Epub 2005 Aug 31. [DOI] [PubMed] [Google Scholar]
- Persson M., Brantefjord M., Hansson E., Rönnbäck L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-α. GLIA. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Jarvis B.W., Takayama K. In: Endotoxin in Health and Disease 687. Brade H., Opal S.M., Vogel S.N., Morrison D.C., editors. Marcel Dekker; New York: 1999. [Google Scholar]
- Saijo K., Winner B., Carson C.T., Collier J.G., Boyer L., Rosenfeld M.G., Gage F.H., Glass C.K. A nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento D., Montorfano I., Cáceres M., Echeverría C., Fernández R., Cabello-Verrugio C., Cerda O., Tapia P., Simon F. Endtoxin-induced vascular endothelial cell migration is dependent on TLR4/NF-κB pathway, NAD(P)H oxidase activation, and transient receptor potential melastatin 7 calcium channel activity. Int. J. Biochem. Cell Biol. 2014;55:11–23. doi: 10.1016/j.biocel.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Skaper S.D., Facci L., Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2013;141:314–327. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. GLIA. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Stienen M.N., Haghikia A., Dambach H., Thöne J., Wiemann M., Gold R., Chan A., Dermietzel R., Faustmann P.M., Hinkerohe D., Prochnow N. Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFβ1 regulation. Br. J. Pharmacol. 2010;162:491–507. doi: 10.1111/j.1476-5381.2010.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-Y., Friedman E., Olmestead M.C., Burns L.H. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gβγ signalling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Wang H.-Y., Burns L.H. Naloxone's pentapeptide binding site on filamin A blocks mu opioid receptor-Gs coupling and CREB activation of acute morphine. Plos One. 2009;4(1):e4282. doi: 10.1371/journal.pone.0004282. Epub 2009 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang X., Zhang Y., Peng Y., Hutchinson M.R., Rice K.C., Yin H., Watkins L.R. Pharmacolocigal characterization of the opioid isomers (+)-naltrexone and (+)-naloxone as Toll-like receptor 4 antagonists. Br. J. Pharmacol. 2016 Mar;173(5):856–869. doi: 10.1111/bph.13394. Epub 2016 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman A., Ginhoux F., Greter M., Bruttger J. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol. 2015;36:625–636. doi: 10.1016/j.it.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Zeng X., Zhang S., Xu L., Yang H., He S. Activation of protease-activated receptor 2-mediated signaling by mast cell tryptase modulates cytokine production in primary cultured astrocytes. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/140812. [DOI] [PMC free article] [PubMed] [Google Scholar]