Abstract
We measured the bulk grain concentrations of arsenic (As), along with rubidium (Rb) and strontium (Sr) as indicators of phloem and xylem transport respectively, in rice (Oryza sativa cv. Italica Carolina) pulsed with arsenate at two exposure levels for 5 day periods at progressively later stages of grain fill, between anthesis and maturity, through the cut flag leaf. We compared these to unexposed (negative) controls and positive controls; pulsed with dimethylarsinic acid (DMA). We collected elemental maps of As and micronutrient elements (Fe, Zn, Mn, Cu and Ni) from developing grains of rice. Exposures were either 25 or 100 μg/ml arsenate (As(V)) at various stages of grain development, compared to 25 μg/ml dimethylarsinic acid (DMA); the most efficiently transported As species identified in rice. We used the spatial distribution of arsenic in the grain to infer the presence of As transporters. By exposing grains through the flag leaf rather than via the roots, we were able to measure arsenic transport into the grain during filling under controlled conditions. Exposure to 100 μg/ml As(V) resulted in widespread As localization in both embryo and endosperm, especially in grains exposed to As at later stages of panicle development. This suggests loss of selective transport, likely to be the result of As toxicity. At 25 μg/ml As(V), As colocalized with Mn in the ovular vascular trace (OVT). Exposure to either As(V) or DMA reduced grain Fe, an effect more pronounced when exposure occurred earlier in grain development. The abundance of Cu and Zn were also reduced by As. Arsenic exposure later in grain development caused higher grain As concentrations, indicating the existence of As transporters whose efficiency increases during grain fill. We conclude that localization of As in the grain is a product of both As species and exposure concentration, and that high As(V) translocation from the flag leaf can result in high As concentrations in the endosperm.
1.0 Introduction
Rice (Oryza sativa L.) is a dietary staple for half of the global population that accumulates the metalloid arsenic (As) [1–11] under flooded soil conditions. Arsenic is a Class I, non-threshold known carcinogen that can cause various cancers (skin, lung, bladder, kidney, liver and prostate) [12], and can affect cardiovascular, hematological, pulmonary, neurological, immunological, and reproductive/developmental function [13] at high chronic exposure levels. As exposure from food to children under the age of three is estimated to be 2–3 fold that of adults [14]. Arsenic exposure from rice in a population with As-free drinking in water in West Bengal (India) has been linked to genotoxicity [15]. Rice-based foods are second only to seafood and seaweed as dietary sources of As [14], but unlike the arsenosugars found in seafood and seaweed [16] (specifically arsenobetaine and arsenocholine), the inorganic forms of As measured in rice are almost 100% bioavailable [17]. The predominant forms of As in rice are the inorganic species (arsenate, As(V) and arsenite, As(III)) and the organic species (dimethylarsinic acid, DMA and monomethylarsonic acid, MMA) [5]; the relative proportions of which are product of both genes and environment [8]. Inorganic As species are more acutely toxic than organic forms, with the median lethal dose of inorganic As several orders of magnitude lower than organic (10–20 μg/ml for As(V) and As(III); 700–2600 for DMA and 700–1600 for MMA)[18]. The order of acute As toxicity is As(III) > As(V) > MMA ≈ DMA, but human carcinogenicity and genotoxicity in humans may not follow this order [18].
As an element with no essential function in plants, As gains entry via the transport systems of essential nutrients phosphorus (P) and silicon (Si)—the phenomenon of molecular mimicry, based on similar chemical properties such as charge and ionic radius[19]. Inorganic As(III) present as arsenous acid (H3AsO3) in flooded rice paddies[20] and enters rice root cell symplast via the silicic acid transporter, Lsi1 (Low Silicon 1) [21, 22], whereas membrane-intrinsic phosphate transport proteins are responsible for As(V) uptake[23]. Organic As species DMA and MMA can also be transported into the root via Lsi1[21]. Arsenite is exported from root cells into the xylem by the effluxer NIP2;1 (nodulin-26 like intrinsic channel proteins) [24], which works in tandem with influxer Lsi1 to shuttle As inwards across the root towards the vasculature [25]. The reduction of As(V) to As(III) governs the transport of As to the edible part of the grain, with almost all As(III) being delivered via the phloem [26, 27].
Arsenite was previously assumed to be the dominant species of inorganic As inside plant cells; however a study of rice exposed to As under Si-depleted conditions found a high proportion of As(V) in the grain [28]. Arsenate continues to use the high affinity P transporters for upward transport into vasculature and leaves[29]. Arsenic is then complexed with glutathione or phytochelatins and sequestered into vacuoles by ABCC-type transporters[30]. Inositol transporters AtINT2 and AtINT4 expressed in the companion cells of the phloem are involved in As(III) loading into the developing grain [31].
In many rice varieties, organic As species constitute a significant proportion of the As in rice, ranging from 26% [32] to 50% [5]. Since plants cannot methylate As, organic As species may be a product of soil microbial activity. Microbial biotransformation of soil As from application of As-rich poultry litter [33] and seaweed fertilizers [33, 34] could theoretically contribute organic As species to the soil. Castlehouse et al.[34] studied the biotransformation of arsenosugars from seaweed fertilizers commonly used in organic farming and found that DMA was the predominant arsenic species in soil porewater. Quantifying the amount and speciation of As in tissues of the rice grain is important for food safety because a wide and increasing number of ingredients are derived from rice, particularly in foods intended for infants, recently been the primary focus of regulatory considerations [35] and gluten-free foods in which rice is the primary grain. Rice products formulated from specific grain layers range from flours and oils to emulsifiers and sweeteners, and can be a major ingredient in a wide range of baked goods [36], infant snacks [37] and powdered milk formulas. The potential of rice products to be significant sources of As exposure was demonstrated recently when organic brown rice syrup (OBRS), used as a sweetener, was found to contain unusually high As concentrations[38]. The accumulation of inorganic As in rice bran [27] means that processed forms of rice bran or whole brown rice may be a significant dietary source of concentrated inorganic As. DMA is efficiently transported into the endosperm, but As(III) remains in the maternally-derived outer bran layers [32], in particular the ovular vascular trace (OVT) a strand of vascular tissue that is the main route of nutrient transport from the mother plant during grain fill. The extent to which As(V) is transported to the grain and its distribution is less clear; obscured by speciation changes that occur during uptake and long distance transport within the plant. The aim of this study was to measured the distribution and abundance of total As in the tissue layers of rice grains pulsed with As(V) directly through the flag leaf during grain development using synchrotron X-ray fluorescence microscopy (SXRF). We comparing this to a negative control (no As pulse) and a positive control consisting of a DMA pulse;the form of As most efficiently transported into the endosperm of rice. Also following our previous work (REF), we used co-exposure to rubidium (Rb) and strontium (Sr) as markers of phloem and xylem transport activity. While bulk analysis of As concentrations in grains is critical, only spatially resolved techniques are able to provide information on the distribution of As between individual tissue layers. Feeding As via the flag leaf as As(V) results in higher grain total As concentration than feeding with As(III) [26]. Here, we show that As(V) when provided to flag leaves at high concentrations can accumulate in the endosperm. Moreover, As accumulation results in changes in the nutrient concentrations and localization in grains. Our results indicate that As species, exposure concentration and grain developmental stage are all important for the final As distribution in grains, with the potential to change nutrient distribution.
2.0 Materials and Methods
2.1 Cultivation of Rice Plants
A quick flowering variety of rice (Oryza sativa L.) cv. Italica Carolina was cultivated in a controlled temperature greenhouse until anthesis, as described in Carey et al.[26]. Briefly, seeds were sown directly into trays of John Innes No. 2 potting compost and transferred to 1 L pots after two weeks under tropical conditions (23–35 °C), with additional lighting supplied at 150 μmol·m−2·s−1 via sodium lamps. Plants were fed with Sangral soluble fertilizer (Sinclair) on a weekly basis, with a N:P:K ratio of 1:1:1 plus 1 MgO diluted 1 in 100 (w/v). Rice panicles were exposed to As(V) or DMA at different stages of grain development as described in Table 1. Treatment stock solutions were prepared by dissolving the appropriate salt in Milli-Q deionized water, and the appropriate number of subsamples of each stock were frozen for subsequent removal and use on the relevant day. Treatment vials were prepared by making 0.25 mL of the defrosted treatment stock up to 1 mL with Milli-Q deionized water and using a 0.1 mL aliquot in a weighed Eppendorf vial, together with MES buffer at a final concentration of 5 mM. Rubidium (Rb) and strontium (Sr) were added as markers for phloem and xylem transport respectively, with final concentration of 85.5 and 87.6 μg/ml (1 mM) respectively[27]. Solutions were pH adjusted to 6.4 with NaOH. The tip of the flag leaf was removed with a surgical steel blade and discarded. The cut surface of the flag leaf was inserted in to the Eppendorf vial, taking care to immerse the cut surface in the treatment solution. Treatment solutions used were As(V) (25 μg/ml and 100 μg/ml) and DMA (25 μg/ml) (Table 1). The experimental design focused on the response of grain development to As(V). Therefore, we included 25 μg/ml DMA treatment at 8 DPA as a qualitative comparison to the As(V) treatments. Our previous work showed that transport of organic As was so efficient that plants would not survive a parallel treatment schedule using DMA [26]. Treatment vials were foil-wrapped to limit evaporative loss and contamination, and the vials were attached using tape. Fresh treatment vials were applied every 24 h, with the tip of the leaf re-cut to ensure entry. Arsenic treatments were applied at varying stages of grain development, as described in Table 1, using the onset of anthesis as a starting point. Grains exposed to 25 or 100 μg/ml As(V) were pulsed immediately after anthesis (0 DPA), or at 5, 10 and 15 DPA, for a total of 5 days, and then harvested at maturity, 20 DPA. Grains exposed to DMA were pulsed at 8 DPA. Treatment vials were frozen on removal from the flag leaf, and three replicate panicles were used.
Table 1.
Concentration of As, Rb (a phloem marker) and Sr (a xylem marker) in rice grains pulsed with arsenate for 5 days during grain fill and harvested at maturity (20 DPA), compared to negative and positive controls.
| As Species | As (mg·L−1) | Timing of 5-day pulse (DPA) | μg·g−1
|
||
|---|---|---|---|---|---|
| As | Rb | Sr | |||
| Negativeb | 0 | 0.3 (0.2) | 2.8 (1.4) | 0.7 (0.5) | |
| Positive | 25 | 8 | 47.2 (2.0) | 9.42 (1.9) | 1.27 (0.1) |
| As V | 25 | 0a | 5.5 (2.4) | 287.3 (142.6) | 116.3 (15.8) |
| 25 | 5 | 4.0 (0.7) | 76.5 (28.2) | 34.1 (10.2) | |
| 25 | 10 | 9.2 (5.0) | 60.0 (24.1) | 9.3 (4.4) | |
| 25 | 15a | 8.2 (2.7) | 20.5 (0.6) | 1.4 (0.5) | |
| As V | 100 | 0 | 7.7 (1) | 62.3 (8.0) | 7.6 (2.7) |
| 100 | 5 | 4.7 (2.2) | 13.2 (3.4) | 1.6 (0.9) | |
| 100 | 10 | 22.7 (0.5) | 36.0 (6.8) | 2.7 (1.0) | |
| 100 | 15a | 17.0 (8.50) | 20.2 (8.4) | 1.0 (0.3) | |
Data are mean (± SD), where N=3.
N=2,
N=6
2.2 ICP-MS Analysis
Treated panicles were analyzed for total As, Rb and Sr via inductively coupled plasma mass spectroscopy (ICP-MS) using an Agilent 7500 instrument (Agilent Technologies, Santa Clara, CA, USA). Grains were selected from the top and middle regions of the panicle and husks were removed manually. Oven dried samples were subject to microwave-assisted digestion and analyzed for total As concentration via ICP-MS as described in Sun et al.[39]. Quality control procedures were as described in Sun et al.[39].
2.3 Synchrotron X-ray Fluorescence Mapping
One fresh grain from one replicate of each treatment was randomly selected for SXRF imaging. Fresh, leaf-fed As-treated grains were analyzed via SXRF mapping at Beamline X26A of the National Synchrotron Light Source (NSLS) of the Brookhaven National Laboratory (Upton, NY, USA). Details of Beamline configuration are as described in Carey et al.[26]. The storage ring at NSLS operated with low emmitance lattice at 300 mA, 2.8 GeV. The X-ray microprobe at X26A used a focused, monochromatic beam with Kirkpatrick-Baez focusing optics capable of focusing the beam down to approximately 5–10 μm. Photon flux at 18 keV is approximately 1 × 109 photons/second. The experimental set up used a Canberra 9-element HPGe Array detector and two Radiant Vortex-EX Silicon Drift Diode detectors. The sample stage sat at 45° to the incident beam, allowing an optical microscope with a gigabit Ethernet CCD attachment to be mounted horizontally for viewing the sample at normal incidence. Analysis was conducted at an incident energy of 12.5 keV to be above the As absorption edge. Elemental mapping of unsectioned rice grains included the bottom half of the grain from endosperm to embryo to maximize efficient use of synchrotron time, because distributions appear consistent throughout the endosperm for our elements of interest [40]. Scanning resolution was standardized for all grains at 12 μm step size and a 0.25 s dwell time.
Element concentrations were calculated for the fluorescence maps as described in McNear et al[41] using measurements of the standard reference materials SRM1832/1833 (National Institute of Standards and Testing) thin-film standards, an assuming an object density of 1.2 g/cm3. SXRF microtomography was also conducted at NSLS beamline X26A on a grain exposed to 25 μg/ml DMA as described in Kim et al.[42], using 12 μm step size, 250 millisecond dwell times, an incident beam energy of 12.5 keV measuring 7 × 10 μm (horizontal × vertical), following techniques described in Carey et al.[26].
2.4 Region of Interest Analysis and Descriptive Statistics
We used the beamline-specific X26A_plot general user interface data analysis software (controlled by the IDL programming language (Excelis Visual Information Solutions, Inc., Boulder, CO)) to trace the borders of the OVT, embryo and endosperm using elements that localized to these areas as markers. We collected the descriptive statistical output for the regions of interest, specifically the total abundances of elements, expressed as normalized fluorescence counts.
3.0 Results and Discussion
3.1 ICP-MS Analysis
Bulk concentrations of As in grains exposed via the cut flag leaf at varying time points prior to anthesis are shown in Table 1. Data are shown for As concentrations in unexposed grains (negative control) and following a 5-day exposure to DMA (positive control).
A 5-day pulse of either 25 or 100 μg/ml As(V) at 10 DPA resulted in higher grain arsenic concentrations. Exposure at 15 DPA resulted in toxicity, which hindered grain development, resulting in lower mean grain arsenic accumulation. As expected, exposure to 100 μg/ml As(V) resulted in higher As accumulation (Table 1, Figure 1). Inclusion of 1 mM Rb and Sr in the pulse solution acted as markers of phloem and xylem transport (respectively), where greater grain concentrations indicate a lack of competitive inhibition as a result of transport of other ions, and lower concentrations indicating competitive inhibition. In As(V)-pulsed grains, both phloem and xylem transport declined with increasing As(V) concentrations as grain fill progressed, suggesting that As(V) transport into the grain involved both vascular systems. The magnitude of phloem transport was comparatively greater than xylem transport throughout, and xylem transport declined to a lower final level, suggesting that As(V) predominantly entered the grain via the phloem. Suppressed Rb grain concentrations in the DMA positive control confirmed earlier findings [27] that DMA uses phloem tissue to enter the grain.
Figure 1.
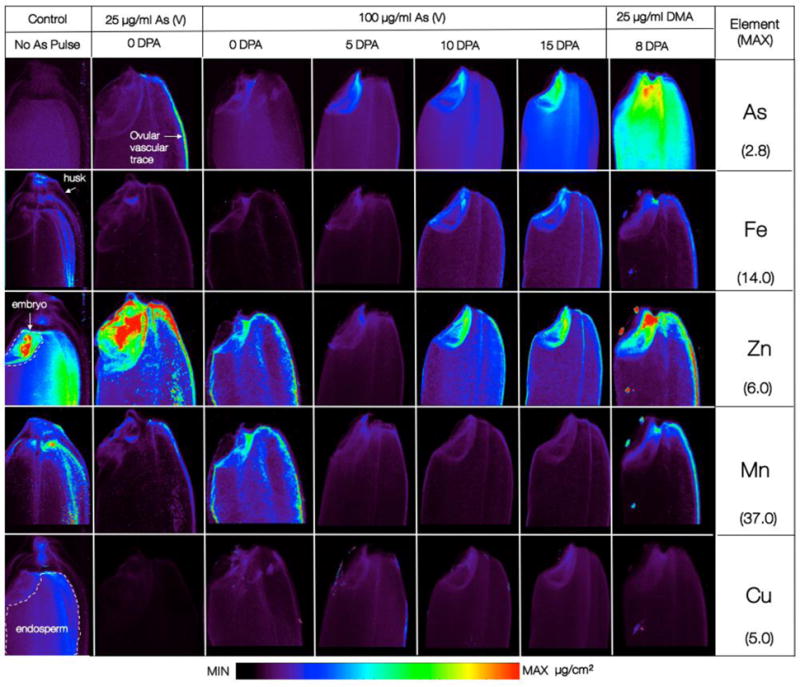
Synchrotron X-ray fluorescence elemental maps of As, Fe, Zn, Mn and Cu in unpolished grains of Oryza sativa cv. Italica Carolina pulsed with As(V) or DMA for 5 days during grain fill. DPA: Days post anthesis. Data are expressed as μg/cm2, using a unified scale for each element. Examples of anatomical features (husk, embryo, endosperm and ovular vascular trace) are indicated.
Overall, we observed elevated phloem and xylem transport corresponding with lower As levels. These data suggest that As(V)-responsive transport is most efficient in the phloem during the later stages of grain development, but also uses the xylem in the early stages of grain fill. Toxicity of As(V) was an issue at these exposure concentrations, resulting in disruption of transport systems and As being detected in the embryo and other layers of the grain. The distribution of As and essential micronutrients in tissues of developing rice grains exposed to As(V) was influenced by As species, concentration and timing of exposure. The higher abundances of As detected in grain tissues exposed to 25 μg/ml DMA at 8 DPA compared to 100 μg/ml As(V) at 0 DPA agree with previous studies on the more efficient transport of DMA in the grain as compared to As(V)[26]. While much of the As(V) reaching higher grain tissues via root uptake is reduced to As(III)[43], studies have detected varying amounts of As(V) in the grain as a minor component ranging from 6.6% of total grain As[44] to almost 18%[45]. The role of genetic variation in As uptake is well-established [[46–48]]. Trans-membrane transport and chemical species changes of As in plants occur at various points along the route from soil to grain, both of which are under genetic control. Because reduction of As(V) in the plant affects delivery of As(V) to the grain, and is itself a variable trait, this study intentionally controlled for As species delivery to the grain, to examine for As(V) and DMA responsive transporters during grain fill.
3.2 Elemental Distribution and Abundance
3.2.1 Elemental Maps
Synchrotron X-Ray Fluorescence elemental maps of As, Fe, Zn, Mn and Cu in grains exposed to As(V) together with positive and negative controls are shown in Figure 1. Elemental maps of low-atomic number elements phosphorus and sulphur were not collected from this dataset due to the use of a higher incident energy needed to reach the As Kα fluorescence line. We did not include calcium or potassium in our analytes of interest because of their high abundance in the husk tissue through which our images were collected. We analyzed the 100 μg/ml treatment only for the As(V) time course because this imparted higher concentrations and more pronounced differences in grain As levels, and because of the limitations on synchrotron beam time. For 2D imaging at 10 μm spatial resolution, the bran layers (pericarp, aleurone and tegument) are not distinguishable. Further, distinguishing the bran layer from the endosperm is also not unequivocal in 2D mapping compared to tomography, therefore we use caution in interpreting two-dimensional map of unsectioned specimens.
Elemental maps showed that grains exposed to 25 μg/ml of As(V) at 0 DPA accumulated As primarily in the OVT, whereas those exposed to 100 μg/ml of As(V) at the same growth stage did not. Bulk grain As concentrations doubled in 100 μg/ml arsenate at 0 DPA compared to 25 μg/ml. Elemental maps indicate that As is diffusely distributed throughout the grain.
Phytotoxicity was a factor in As distribution at 100 μg/ml As(V). Exposure to 100 μg/ml As(V) at all tested timepoints resulted in As accumulation in the embryo, endosperm or bran layer, rather than solely the OVT. Exposure at later stages of grain development resulted in higher As accumulation (Figure 1). Arsenic distribution in the DMA positive control confirmed previous findings that as DMA, As can access the embryo and endosperm or bran layer of the grain, and bypass the OVT, where As(III) is usually retained [26].
We infer that transporters for As(V) exist throughout the grain, and that their steady state levels change during development and their capacity for transport is modified by As toxicity. Higher abundances of elements in tissue layers may indicate inefficient transport (where elements are not being transported beyond that point). Lower concentrations may indicate efficient transport or sequestration in tissues closer to the source, such as the flag leaf. In rice grains, the vascular traces, particularly the OVT, move mineral nutrients from the flag leaf of the mother plant toward their respective end points in the embryo and bran layers. Results of grain exposure to 25 μg/ml As(V) at the beginning of grain development in comparison with 100 μg/ml As(V) later in development may indicate that accumulation of As(V) in rice grain hinges upon efficient transport by the OVT, which contained the highest As abundances following early treatment with 25 μg/ml As(V) treatment. This also suggests that transport beyond this point to the embryo and endosperm is inefficient. This agrees with previous studies that have focused on inorganic As(III) remobilization during grain fill[26]. When As(V) was introduced later, even at four times the concentration, accumulation in the OVT was not observed, embryo abundances were higher and endosperm As levels are approximately equal.
Grains pulsed with As(V) later in grain development had higher relative abundances of Fe in the embryo and lateral vascular trace than those exposed earlier in development, as well as control grains. Given the toxicity of this treatment, it is likely that variations in Fe stem from disruptions in nutrient transport. Zinc abundances were consistent between grains from different exposure scenarios, distributed primarily in the embryo and tissues of the OVT. Manganese (Mn) and Zn distribution and abundance were disrupted by As exposure; compared to with negative control grains. Grain exposed to 100 μg/ml of As(V) early in grain development contained comparable Mn levels to controls with indications of aleurone-wide distribution but grains exposed to 100 μg/ml As(V) later on in grain development showed lower concentrations of Mn. Negative control grain indicated Mn in the aleurone and in the OVT, consistent with previous studies[26], whereas positive control DMA-exposed grains had strong Mn localization in the OVT, and comparatively less aleurone-associated Mn. This may indicate that preferential transport of DMA inhibits centripetal Mn transport out of the OVT.
The abundance of Cu was low and distribution was diffuse throughout the endosperm. Although grain-to-grain variation cannot be discounted, grains pulsed with 25 μg/ml As(V) at 0 DPA contained Cu that fell below SXRF detection limits.
These findings suggest that at sub-toxic concentrations, As(V) is transported via the OVT, in common with As(III). The higher concentration of As(V) used in the experiment is suspected to have overwhelmed the transporters in the OVT, disrupting normal membrane transport selectivity and allowing As to enter the endosperm and embryo.
3.2.2 Tomography
SXRF microtomography of grains exposed to DMA are shown in Figure 2. Arsenic was distributed both in the bran layer and endosperm. The highest abundances of As were in the bran layer, and then progressively lower As abundances were observed with increasing distance from the bran layer, consistent with the centripetal movement of mineral nutrients from the bran layer into the endosperm[49]. Other elements (Fe, Zn, and Mn) were present only in the bran layer, and showed no indication of movement beyond this layer.
Figure 2.
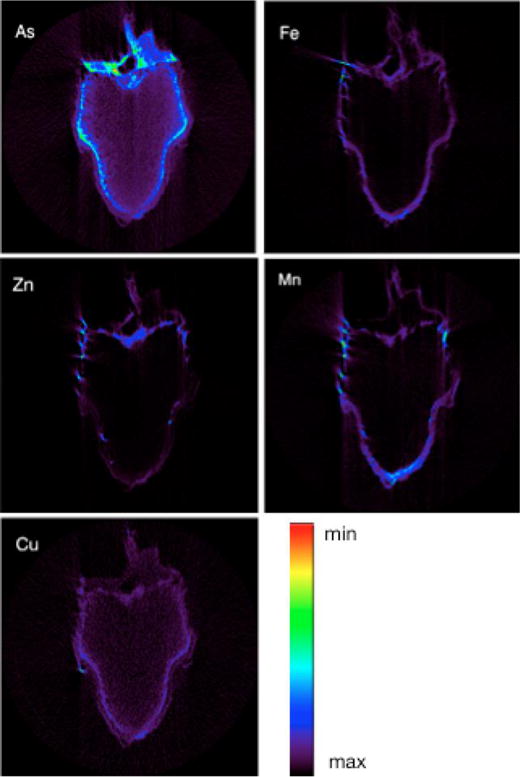
SXRF tomograms of rice grains exposed to 25 μg/ml of DMA at 8 DPA. Data are normalized fluorescence counts.
3.2.3 User-defined Region-of-Interest Analysis
Total fluorescence counts of As, Fe, Zn, Mn and Cu in the embryo, endosperm and OVT are shown in Figures 3. Grains exposed to 25 μg/ml of As(V) at 0 DPA had the highest abundances of As in the OVT; approximately four times higher than treatment with 25 μg/ml DMA at 8 DPA. Exposing grains to 100 μg/ml As(V) later in grain development resulted in greater abundances of As in the OVT compared to earlier exposure, although levels did not reach those observed in exposure to 25 μg/ml of As(V).
Figure 3.
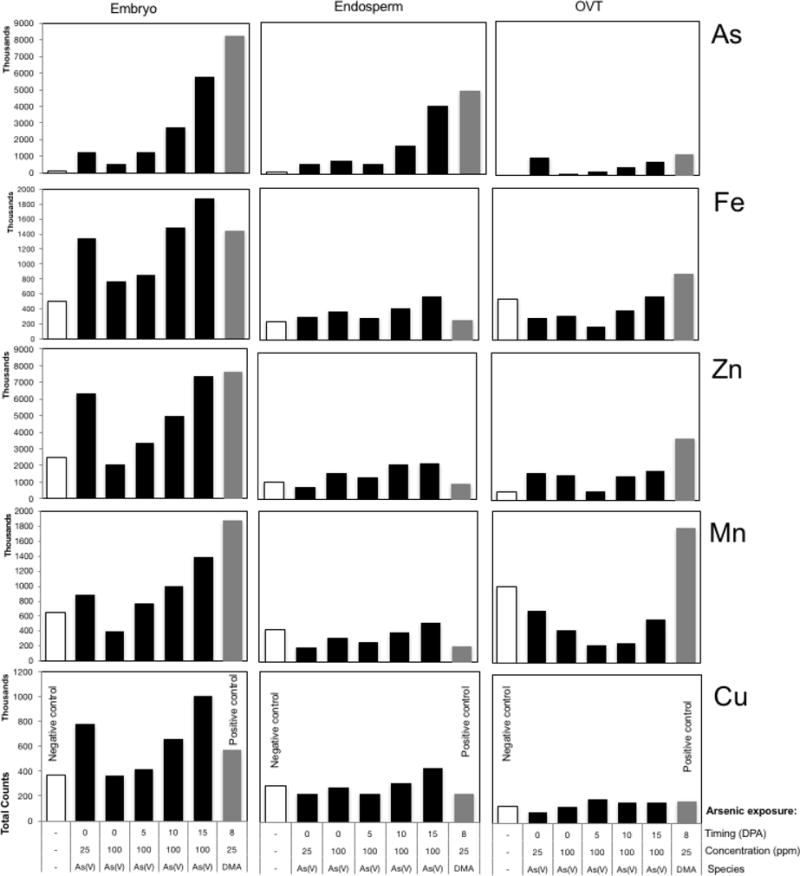
Total fluorescence counts in user-defined region of interest analysis of As, Fe, Zn, Mn, and Cu in the embryo, endosperm and OVT of rice grains exposed to As(V) or DMA
The OVT contained comparatively higher abundances of Fe, Mn and Zn and lower abundances of Cu. Treatment with As (V) during grain development reduced the abundance of Fe and Mn in the OVT, but did not appear to suppress Zn or Cu. Mean Zn abundances in the OVT were relatively consistent across treatments, with the exception of early treatment with 25 μg/ml As(V), where OVT Zn abundances were higher.
For all elements including As, the highest total counts were observed in the embryo of the grain. Treatment with 100 μg/ml As(V) progressively later in development resulted in progressively greater As abundances in the embryo. This same trend of increasing abundances with later As treatment was observed for Fe, Zn Mn, and Cu. In each case, treatment with 25 μg/ml As(V) at 0 DPA results in an elevated abundance relative to treatment with 100 μg/ml, and the later the treatment, the higher the abundance. These observations are likely due to increasing toxicity in response to the higher concentration of As(V). Exposure to the lower As concentration earlier in grain fill caused less damage to translocation system function between leaf and grain, seen in elevated concentrations of metals. Xylem transport declines as grain fill progresses (Table 1), but phloem transport continues to convey elements to the grain, where As toxicity affects grain development, and transport systems that convey essential nutrients to the outer layer of the endosperm are disrupted. Following DMA treatment, abundances of Fe, but not Zn and Mn were lower. Arsenic was detected in the endosperm as a result of treatment both with As(V) and DMA (Figure 3), but total counts were lower in the endosperm than in the embryo. In 2D maps, elemental accumulation in the bran layers cannot be distinguished beyond doubt from the inner endosperm. However, abundances confirm the trend observed in other tissue areas: treatment with 25 μg/ml As(V) early in grain fill results in higher As abundances; treatment with 100 μg/ml As(V) progressively later in grain fill result in progressively higher abundances, and that exposure to 25 μg/ml DMA results in higher abundances than As(V). Abundances of Fe, Mn and Zn in the starchy endosperm are typically low, and any of the elements detected are likely to arise from the peripheral bran layers. We observed a positive trend with Fe (mean abundances increased with progressively later treatment with 100 μg/ml As(V) and with 25 μg/ml DMA), but observed a negative trend with Zn, Mn, Cu and Ni.
4.0 Conclusions
In this study, we used presence of an element in a specific grain tissue layer as evidence for metal transport. Expression of metal transporter genes and XRF-based metal localization in rice seeds has also been used to identify putative transporters involved in metal movement during initial phases of germination [50]. Our findings are that As(V) transport into the endosperm is less efficient than DMA, but efficiency increases during grain fill at the expense of Fe and Mn transport. Zheng et al.[51] also found similar temporal variation in As species in the various tissues of rice grain, with increasing inorganic As species as development progressed.
HIGHLIGHTS.
Developing rice grains were exposed to arsenate via flag leaf during development
Exposure later in development caused higher grain As concentrations
At 25 μg/ml, arsenic co-localized with manganese in the ovular vascular trace
Exposure recued grain Fe, Cu and Zn
Acknowledgments
Use of the National Synchrotron Light Source, beamline X26A at the Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. This work was supported by funding from the National Institute of Environmental Health Sciences Superfund Basic Science Research Program to TP grant number P42007373, by the UK Biotechnology and Biological Sciences Research Council to AMC, and by National Science Foundation Plant Genome grant DBI 0701119 to FKR. FKR was also supported by a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: T.P. and F.K.R carried out the SXRF analysis, data processing and writing, A.-M.C. carried out the rice growth and exposure experiments and A.A.M. is the Principal Investigator of the study.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Abedin JMD, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. Arsenic accumulation and metabolism in rice (Oryza sativa L.) Environmental Science and Technology. 2002;36(5):962–968. doi: 10.1021/es0101678. [DOI] [PubMed] [Google Scholar]
- 2.Abedin JMD, Feldmann J, Meharg AA. Uptake kinetics of arsenic species in rice plants. Plant Physiology. 2002;128:1120–1128. doi: 10.1104/pp.010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meharg AA, Rahman M. Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environmental Science and Technology. 2003;37(2):229–234. doi: 10.1021/es0259842. [DOI] [PubMed] [Google Scholar]
- 4.Meharg AA. Arsenic in rice - understanding a new disaster for South-East Asia. Trends in Plant Science. 2004;9(9):415–417. doi: 10.1016/j.tplants.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Williams PN, Price AH, Raab A, Hossain MA, Feldmann J, Meharg AA. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environmental Science and Technology. 2005;39(15):5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- 6.Meharg A, Sun G, Williams PN, Adomako EE, Deacon C, Zhu YG, Feldmann J, Raab A. Inorganic arsenic levels in baby rice are of concern. Environmental Pollution. 2008;152:746–749. doi: 10.1016/j.envpol.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Meharg AA, Deacon C, Campbell RCJ, Carey AM, Williams PN, Feldmann J, Raab A. Inorganic arsenic levels in rice milk exceed EU and US drinking water standards. Journal of Environmental Monitoring. 2008;10:428–431. doi: 10.1039/b800981c. [DOI] [PubMed] [Google Scholar]
- 8.Norton GJ, Pinson SRM, Alexander J, McKay S, Hansen H, Duan GL, Islam MR, Ismam S, Stroud JL, Zhao FJ, McGrath SP, Zhu YG, Lahner B, Yakubova E, Guerinot ML, Tarpley L, Eizenga GC, Salt DE, Meharg AA, Price AH. Variation in grain arsenic assessed in a diverse panel of rice (Oryza sativa) grown in multiple sites. New Phytologist. 2012;193(3):650–664. doi: 10.1111/j.1469-8137.2011.03983.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao FJ, Zhu YG, Meharg AA. Methylated Arsenic Species in Rice: Geographical Variation, Origin, and Uptake Mechanisms. Environmental Science & Technology. 2013;47(9):3957–3966. doi: 10.1021/es304295n. [DOI] [PubMed] [Google Scholar]
- 10.Mitani N, Chiba Y, Yamaji N, Ma JF. Identification and Characterization of Maize and Barley Lsi2-Like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell. 2009;21(7):2133–2142. doi: 10.1105/tpc.109.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punshon T, Jackson BP, Meharg AA, Warczack T, Scheckel K, Guerinot ML. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Science of The Total Environment. 2017;581-582:2009–220. doi: 10.1016/j.scitotenv.2016.12.111. 1 March 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ATSDR. Toxicological Profile for Arsenic. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2007. [PubMed] [Google Scholar]
- 13.WHO. Safety evaluatrion of certain contaminants in food. World Health Organization; Rome, Italy: 2011. p. 800. [Google Scholar]
- 14.European Food Safety Authority, E. EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on Arsenic in Food. EFSA Journal. 2009;7(10):1351–198. [Google Scholar]
- 15.Banerjee M, Banerjee N, Bhattacharjee P, Mondal D, Lythgoe PR, Martinez M, Pan J, Polya DA, Giri AK. High arsenic in rice is associated with elevated genotoxic effects in humans. Sci Rep. 2013;3:2195. doi: 10.1038/srep02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen EH, Pritzl G, Hansen SH. Arsenic speciation in seafood samples with emphasis on minor constituents: An investigation using High-Performance Liquid Chromatography with detection by Inductively Coupled Plasma Mass Spectrometry. Journal of Analytical Atomic Spectrometry. 1993;8:1075–1084. [Google Scholar]
- 17.Meharg AA, Zhao FJ. Arsenic & rice. Dordrecht: Springer Verlag; 2012. p. xi, 171. [Google Scholar]
- 18.Le XC, Ma M, Lu X, Cullen WR, Aposhian HV, Zheng B. Determination of monomethylarsonous acid, a key arsenic methylation intermediate, in human urine. Environmental Health Perspectives. 2000;108(11):1015–1018. doi: 10.1289/ehp.001081015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicology and Applied Pharmacology. 2005;204(3):274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu XY, McGrath SP, Meharg AA, Zhao FJ. Growing rice aerobically markedly decreases arsenic accumuulation. Environmental Science and Technology. 2008;42:5574–5579. doi: 10.1021/es800324u. [DOI] [PubMed] [Google Scholar]
- 21.Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiology. 2009;150:2071–2080. doi: 10.1104/pp.109.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytologist. 2010;186:392–399. doi: 10.1111/j.1469-8137.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 23.Quaghebeur M, Rengel Z. Arsenic uptake, translocation and speciation in pho1 and pho2 mutants of Arabidopsis thaliana. Physiologia Plantarum. 2004;120:280–286. doi: 10.1111/j.0031-9317.2004.0240.x. [DOI] [PubMed] [Google Scholar]
- 24.Pommerrenig B, Diehn TA, Bienert GP. Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci. 2015;238:212–27. doi: 10.1016/j.plantsci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature. 2007;448:209–212. doi: 10.1038/nature05964. 12 July 2007. [DOI] [PubMed] [Google Scholar]
- 26.Carey AM, Norton GJ, Deacon C, Scheckel KG, Lombi E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA. Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytologist. 2011;192(1):87–98. doi: 10.1111/j.1469-8137.2011.03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA. Grain unloading of arsenic species in rice. Plant Physiology. 2010;152(1):309–319. doi: 10.1104/pp.109.146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seyfferth AL, Webb SM, Andrews JC, Fendorf S. Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochimica et Cosmochimica Acta. 2011;75:6655–6671. [Google Scholar]
- 29.Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annual Review of Plant Biology. 2010;61:7.1–7.25. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 30.Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee Y, Martnoia E. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proceedings of the National Academy of Sciences. 2010;107(49):21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan GL, Hu Y, Schneider S, McDermott J, Chen J, Sauer N, Rosen BP, Daus B, Liu Z, Zhu YG. Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nature Plants. 2015 doi: 10.1038/nplants.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombi E, Scheckel KG, Pallon J, Carey AM, Zhu YG, Meharg AA. Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytologist. 2009;184:193–201. doi: 10.1111/j.1469-8137.2009.02912.x. [DOI] [PubMed] [Google Scholar]
- 33.Jackson BP, Bertsch PM, Cabrera ML, Camberato JJ, Seaman JC, Wood CW. Trace element speciation in poultry litter. Journal of Environmental Quality. 2003;32(2):535–540. doi: 10.2134/jeq2003.5350. [DOI] [PubMed] [Google Scholar]
- 34.Castlehouse H, Smith C, Raab A, Deacon C, Meharg AA, Feldmann J. Biotransformation and accumulation of arsenic in soil amended with seaweed. Environmental Science & Technology. 2003;37(5):951–957. doi: 10.1021/es026110i. [DOI] [PubMed] [Google Scholar]
- 35.FDA. Arsenic in rice and rice products. Foodborne Illnesses and Contaminants. 2016 [cited 2016 February 10 2016] [Google Scholar]
- 36.Juliano BO. In: Rice in human nutrition. F.a A.O.o.t U Nations, editor. FAO and Information Network on Post-Harvest Operations; 1993. [Google Scholar]
- 37.Karagas MR, Punshon T, Sayarath V, Jackson BP, Folt CL, Cottingham KL. Association of Rice and Rice-Product Consumption With Arsenic Exposure Early in Life. JAMA Pediatr. 2016;170(6):609–616. doi: 10.1001/jamapediatrics.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson BP, V, Taylor F, Cottingham KL, Punshon T. Arsenic concentration and speciation in infant formulas and first foods. Pure and Applied Chemistry. 2011;84:215–224. doi: 10.1351/PAC-CON-11-09-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun GX, Williams PN, Zhu YG, Deacon C, Carey AM, Raab A, Feldmann J, Meharg A. Survey of arsenic and its speciation in rice products such as breakfast cereals, rice crackers and Japanese rice condiments. Environment International. 2008;35(3):473–475. doi: 10.1016/j.envint.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Oli P, Ward R, Adhikari B, Mawson AJ, Adhikari R, Wess T, Pallas L, Spiers K, Paterson D, Torley P. Synchrotron X-ray Fluorescence Microscopy study of the diffusion of iron, manganese, potassium and zinc in parboiled rice kernels. LWT - Food Science and Technology. 2016;71:138–148. [Google Scholar]
- 41.McNear D, Peltier E, Everhart J, Chaney R, Sutton S, Newville M, Sparks DL. Application of quantitative fluorescence and absorption-edge computed microtomography to image metal compartmentalization in Alyssum murale. Environmental Science and Technology. 2005;39:2210–2218. doi: 10.1021/es0492034. [DOI] [PubMed] [Google Scholar]
- 42.Kim SA, Punshon T, Lanzirotti A, Liangtao L, Alonso JM, Ecker JR, Kaplan J, Guerinot ML. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science. 2006;314(5803):1295–1298. doi: 10.1126/science.1132563. [DOI] [PubMed] [Google Scholar]
- 43.Raab A, Williams PN, Meharg A, Feldmann J. Uptake and translocation of inorganic and methylated arsenic species by plants. Environmental Chemistry. 2007;4(3):197–203. [Google Scholar]
- 44.Huang JH, Fecher P, Ilgen G, Hu KN, Yang J. Speciation of arsenite and arsenate in rice grain - Verification of nitric acid based extraction method and mass sample survey. Food Chemistry. 2012;130(2):453–459. [Google Scholar]
- 45.Batista BL, Souza JMO, De Souza SS, Barbosa F. Speciation of arsenic in rice and estimation of daily intake of different arsenic species by Brazilians through rice consumption. Journal of Hazardous Materials. 2011;191(1-3):342–348. doi: 10.1016/j.jhazmat.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 46.Norton GJ, Deacon C, Xiong L, Huang S, Meharg AA, Price AH. Genetic mapping of the rice ionome in leaves and grain: identification of QTS for 17 elements including arsenic, cadmium, iron and selenium. Plant and Soil. 2010;329:139–153. [Google Scholar]
- 47.Norton GJ, Douglas A, Lahner B, Yakubova E, Guerinot ML, Pinson SR, Tarpley L, Eizenga GC, McGrath SP, Zhao FJ, Islam MR, Islam S, Duan G, Zhu Y, Salt DE, Meharg AA, Price AH. Genome wide association mapping of grain arsenic, copper, molybdenum and zinc in rice (Oryza sativa L.) grown at four international field sites. PLoS One. 2014;9(2):e89685. doi: 10.1371/journal.pone.0089685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norton GJ, Lou-Hing DE, Meharg AA, Price AH. Rice-arsenate interactions in hydroponics: whole genome transcrpitional analysis. Journal of Experimental Botany. 2008;59(8):2267–2276. doi: 10.1093/jxb/ern097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan S, Dayanandan P. Structural and histochemical studies on grain-filling in the caryopsis of rice (Oryza sativa L.) Journal of Bioscience. 2003;28(4):455–469. doi: 10.1007/BF02705120. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M, Nozoye T, Kitajima N, Fukuda N, Hokura A, Terada Y, Nakai I, Ishimaru Y, Kobayashi T, Nakanishi H, Nishizawa NK. In vivo analysis of metal distribution and expression of metal transporters in rice seed during germination process by microarray and X-ray Fluorescence Imaging of Fe, Zn, Mn, and Cu. Plant and Soil. 2009;325(1-2):39–51. [Google Scholar]
- 51.Zheng MZ, Cai C, Sun GX, Williams PN, Cui HJ, Li G, Zhao FJ, Zhu YG. Spatial distribution of arsenic and temporal variation of its concentration in rice. New Phytologist. 2011;189:200–209. doi: 10.1111/j.1469-8137.2010.03456.x. [DOI] [PubMed] [Google Scholar]


