Fig. 4.
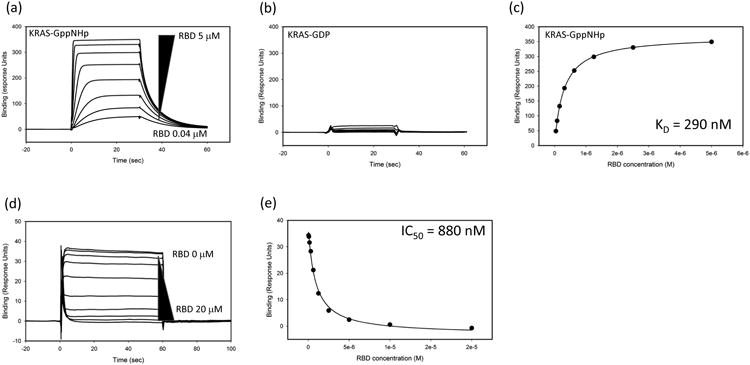
Surface Plasmon Resonance (SPR) analysis of KRAS:RBD interactions. Increasing concentrations of RAF1-RBD (5 – 0.04 uM; 2-fold dilutions) were flowed over KRAS-GppNHp (panel a) or KRAS-GDP (panel b). The binding response to KRAS-GppNHp can be fit to determine the equilibrium dissociation constant (KD) of the interaction (panel c). Inhibition of the interactions can be measured using variable amounts (20 – 0 uM; 2-fold dilutions) of Avi-RBD incubated with 300 nM of KRAS-GppNHp. In this case, unbound KRAS-GppNHp binds to captured Avi-RBD on the chip surface (panel d). Panel e shows the inhibition profile and calculation of an IC50 value.
