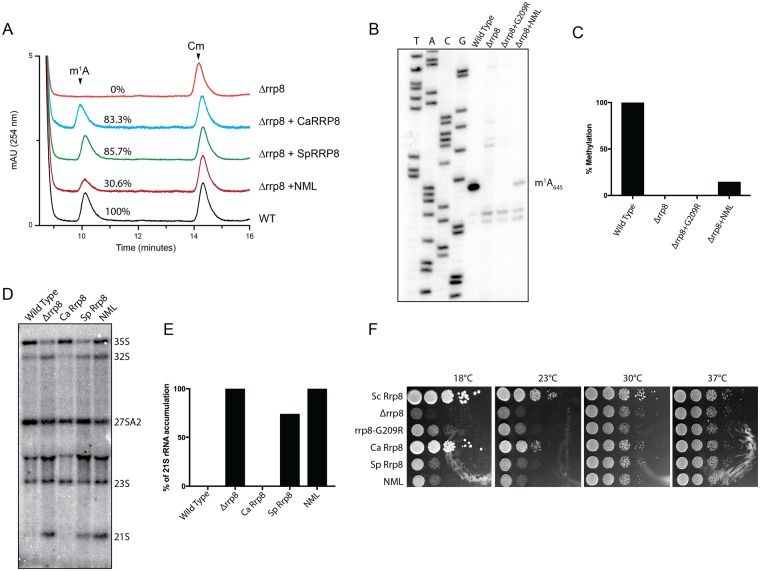
Figure 2.
Heterologous expression of RRP8 homologs and biochemical and phenotypic analysis. (A) Overlaid RP-HPLC chromatograms of the nucleosides derived from fragments isolated using mung bean nuclease assay, containing m1A645 (Sc-Oligo645) isolated from wild-type (WT), deletion mutant (Δrrp8) or deletion mutant strains expressing homologs of RRP8 (CaRRP8 for C. albicans Rrp8, SpRRP8 for S. pombe Rrp8 and NML for H. sapiens Rrp8/NML). (B) Primer extension analysis of Δrrp8, the loss of methylation mutant rrp8G209R and the heterologous expressed human homolog NML. The presence of m1A at position 645 in the 25S rRNA from the wild-type cells led to a strong stop. The expression of the human homolog NML in the deletion mutant of RRP8 of S. cerevisiae also led to a stop at this position to an extent of about 40% compared with S. cerevisiae wild-type. (C) Percent methylation was quantified from the intensity of primer extension stop (panel B) using ImageJ software (http://imagej.nih.gov/ij/). (D) Northern blot analysis of RRP8 homologs expressed in S. cerevisiae. 5 µg of isolated total RNA was separated on an 1.2% agarose-gel and subsequently blotted onto a Hybond H+ nylon membrane. The membrane was hybridized with radioactive (32P)-labeled probes against the internal transcribed spacer 1 (ITS1) (region between cleavage sites A2-A3). (E) Percent of 21S rRNA accumulation in panel D was quantified using ImageJ software. (F) Ten-fold dilutions of strains expressing RRP8, RRP8 homologs or catalytically-defective mutants. Cells spotted onto solid YEPD plates were incubated at four different temperatures illustrating the cold temperature sensitive phenotype of Δrrp8. The differences in the methylation and complementation of Rrp8 homologs of other fungal species and human cells are likely due to sequence specificities that may influence how efficiently a heterologous protein is assembled within the ribosome assembly machinery of the host cell. For example, the S. pombe protein may be incorporated in maturing subunits sufficiently well to restore methylation but not well enough to suppress the RNA cleavage defect, while the C. albicans protein may be recruited to pre-ribosomes more efficiently, allowing it to suppress both defects.