Abstract
Background
Mothers have been shown to have higher morning cortisol on days they go to work compared to non-workdays; however, it is unknown how maternal workday associates with child morning cortisol or the attunement of mother-child morning cortisol.
Aims
This study examined the presence and stability of morning cortisol levels and slopes (i.e., cortisol awakening response or CAR) in a sample of 2 – 4 year old children in out-of-home child care with working mothers. In addition, we examined the differential contributions of maternal workday on mother-child attunement in morning cortisol.
Method
Mother and child morning cortisol was sampled twice a day (awakening and 30 minutes later) across four consecutive days (2 non-workdays; 2 workdays) among 47 working mothers and their young children. Mothers also reported on compliance with sampling procedures and provided demographic information.
Results
While children exhibited stability in cortisol levels, children’s CARs were variable, with children’s non-work CARs not predictive of work CARs. Similarly, a significant morning rise in cortisol was only found on workdays, not non-workdays. Overall, mothers had higher cortisol levels and steeper CARs than their children. Further, maternal workday moderated the attunement of mother-child CARs, such that mothers and children had concordant cortisol levels on non-workdays, but discordant cortisol levels on workdays.
Conclusions
Morning cortisol may be more variable in pre-school aged children than adults but may be similarly responsive to the social environment. Further, workday mornings may be a time of reduced mother-child cortisol attunement.
Keywords: cortisol awakening response, synchrony, attunement, concordance, cortisol, hypothalamic pituitary adrenal axis, mothers, children
Introduction
An emerging literature has highlighted morning cortisol levels and change across the morning (i.e., the cortisol awakening response or CAR) as indicators of chronic stress, and long-term mental and physical health [1]. This literature has focused on adults, and great strides have been made in understanding the factors that explain variability in morning cortisol [2]. For example, work schedules must be assessed given the higher morning cortisol levels [3], and steeper CARs [4] on workdays compared to non-workdays. The production and release of cortisol in children is also sensitive to context [5–6], however few studies have examined daily variation in morning cortisol during early childhood. The majority of pre-school aged children have a clear difference in daily routine based on work and weekend days. These systematic differences in morning routines have the potential to add unexplained variance in child morning physiology, yet to the best of our knowledge, no study has examined workday/non-workday differences in the morning cortisol of children of working mothers. Further, mother and child cortisol levels have been found to be moderately to strongly correlated [7], with maternal cortisol levels as strong of a predictor of child cortisol as children’s behavioral profiles [8]. Given the documented effect of workday on maternal cortisol, there is the potential that workday may also lead to an “un-coupling” of mother-child shared physiology. Therefore, this study employs a “biosocial family systems” approach [9] to examine the relationships between maternal workday, child morning cortisol, and the coordination/attunement between mother and child morning cortisol across days.
Psychosocial stress translates into physiological change through the hypothalamic-pituitary-adrenal (HPA) axis and subsequent cortisol production. Cortisol functions to increase the availability of glucose, thus preparing the body to face perceived challenges [10]. Cortisol is released across the day following a distinct rhythm marked by a rise in the early morning and a decline across the afternoon. Super-imposed on this rhythm is a normative increase in cortisol across the first 30 to 45 minutes post-awakening [11]. Studies of adults have found cortisol levels to increase roughly 50 – 75% across this timeframe [12]. An emerging literature has linked dysregulations in morning cortisol in adulthood to worse health outcomes [2]. Furthermore, studies of acute HPA reactivity suggest early experiences program the set point and threshold of reactivity of HPA axis functioning, providing a mechanism by which early experiences are translated into adult health outcomes [13]. Taken together, this literature suggests the importance of examining early environments in relation to children’s morning cortisol.
Infants develop a diurnal rhythm that consists of relatively high morning and low evening cortisol levels by three months of age [14–15]. However, the CAR is a physiologically distinct phenomenon from the diurnal rhythm [11] and findings have been mixed regarding when a consistently occurring CAR emerges. Three recent studies have found a significant rise in cortisol levels across the first 30 minutes post-awakening, in infants in the first 6 months postpartum [16], in infants between 2 and 12 months [17], and in a highly controlled study of seven 2 – 4 year olds [18]. However, other researchers failed to capture a morning rise in cortisol among 7 – 17 month old infants on a single day of saliva collections by the mother [8]. Further, even studies that have found an overall increase in cortisol across awakening have noted significant variability in morning cortisol across days [17], with a decrease in cortisol across almost 40% of the mornings [16]. The day-to-day variability in child morning cortisol raises the possibility that daily changes in the child’s environment may be related to cortisol levels and awakening responses.
Numerous studies utilizing laboratory stress tasks and reactivity paradigms have linked multiple family interaction patterns and family contexts to child cortisol reactivity and regulation [19–20]. Relatively less attention has been given to understanding naturally occurring fluctuations in daily cortisol levels among young children in their home environment [21]. Despite the paucity in research, two studies suggest children’s daily cortisol in the home is related to the social context of the home. Specifically, higher maternal parenting quality [22] and fewer parent-child conflicts [23] have been related to steeper cortisol declines across the afternoon. Yet these findings are not necessarily generalizable to morning cortisol, given the distinct physiological processes that occur in the morning (i.e., the cortisol awakening response),
Stress physiology is highly sensitive to social influence and in particular, early familial and parent-child interactions [19–20]. Thus, day-to-day changes in family experiences are likely to be linked to daily fluctuations in child cortisol [23]. While families are composed of numerous, ever-changing interactions [24], many with the potential to be related to cortisol [20], this study focuses on maternal work schedule to provide a systematic and recurring family experience, and its relation to variation in morning cortisol. From a behavioral perspective, the highly concentrated family interactions that occur in the morning might be particularly important for dual-earner families who spend a large portion of their waking hours apart [3]. Additionally, [25] and reduce maternal emotional engagement with her children [26]. Further, biosocial studies have consistently linked workday to morning cortisol in adults [4] and, among mothers of pre-school aged children, workday stressors have been found to affect not only maternal cortisol levels but also the cortisol levels of other family members [27]. Thus, we hypothesize that the differential routines and behaviors that are expressed on maternal workdays will lead to child cortisol being higher on maternal workdays than non-workdays.
Recent studies have revealed maternal cortisol to be an important predictor of child cortisol in the morning [8], across the day [28], and in response to challenge [7,29, 30]. While the previously cited studies span a large developmental age range, we acknowledge that mother-child physiological attunement at each stage of development may have unique developmental implications for the mother-child dyad. However, one unifying implication of these studies suggests that in addition to members of the dyad influencing one another’s behavior, emotions, and physiology [7, 31], shared environments or emotions can be translated into shared physiological levels (i.e., mutual or parallel coordination/attunement) [7]. For example, Papp and colleagues [28] found that dyads who engaged in more shared activities had a stronger relationship between mother and child cortisol across the day than dyads who engaged in fewer shared activities. Inherent in this framework is the idea that mother-child physiological coordination is not a characteristic of the dyad, but dependent on the cognitions, behaviors, and social context of the dyad. However, past studies examining mother-child physiological coordination have focused on between-dyad differences, as opposed to examining how mother-child concordance might fluctuate within a dyad [29]. Given the known relationship between work and parental morning cortisol [3], and the potential for work stress to spill over into other family members’ physiology [27], we will examine how the attunement of mother-child morning cortisol changes across maternal non-work and workdays.
Current estimates reveal 64.2% of mothers with pre-school aged children are participating in the labor force [32]. Correspondingly, reports show 86% of children between birth and age 4 with working mothers are cared for by non-relatives while their mothers work [33]. Thus, maternal work schedules have the potential to play a role in the majority of young children’s day-to-day morning cortisol, as well as influence the morning cortisol attunement of many mother-child dyads. We hypothesize that for both mothers and children, non-workday morning cortisol will positively predict workday morning cortisol. However, we expect that the interactions and routines required of mothers and children on days the dyad is leaving the home for work and child care will increase cortisol levels and steepen awakening responses compared to non-workday mornings. Further, mothers’ morning cortisol will predict children’s morning cortisol; however, we expect workday to moderate this relationship, such that mother and child morning cortisol will not be attuned on workdays when mothers are mentally and physically preparing for their workday.
Method
Participants
Fifty-six working mothers and their pre-school aged children were recruited through fliers posted in local child care centers and public places. Of these dyads, 47 children were cared for outside of the home. Because we hypothesize that linkages between maternal workday and child cortisol attunement may be driven, in part, by the routines involved in the dyad leaving the house for work and non-maternal care, we excluded families of working mothers whose children were cared for by in-home caregivers. Eligibility criteria required that mothers were employed outside the home, were the biological mother of a child between the ages of 2 and 4 (M = 3.51 years, SD = .90) who attended out-of-home child care, and that both mother and child were free of chronic illnesses. Pregnant mothers were excluded regardless of trimester.
We focus on biological mother-child dyads with children between 2–4 years old for several reasons. Children in this age range are old enough that they are typically no longer breastfed, and therefore not directly exposed to maternal physiology. However, these children are young enough that they are not in compulsory schooling, thus their schedules are more likely dictated by family routines as opposed to the timing of public education. Further, mothers tend to be primary caregivers [34] and are more likely to take primary responsibility for readying their children for the upcoming day. Additionally, we opted to limit our examination to biological mothers to control for heritability. Thus, to be more confident in reports of biological parenthood and reduce variability in parent-child cortisol attunement, we limited our study to mothers [35].
Mothers ranged in age from 22 to 43 years (M = 31.15 years, SD = 4.85), and the majority (74.5%) were married at the time of this study. The majority of mothers (80.4%) reported their ethnicity as non-Hispanic white and 75% percent of the mothers reported their total combined annual household income at or below $89,999. Fifty-seven percent (57.3%) of the mothers worked 40 hours per week or more and 97.9% worked 20 hours or more. Only one mother worked less than 20 hours a week (M = 36.62 hr, SD = 8.98, range: 11 – 60 hours/week). Correspondingly, 76.5% of the children spent 40 hours per week or more in non-parental care (M = 37.27 hr, SD = 11.51, range: 10 – 64 hours/week) with one child spending under 20 hours in non-parental care.
Procedure
The current study was approved by the Institutional Review Board at Purdue University that oversees ethical concerns for research with human subjects. Prior to partaking in the study, mothers gave informed consent for both their participation and that of their child. Mothers met individually with research assistants to review the study protocol and learn saliva collection techniques at the time of consent. Research assistants also gave participants instructions on collecting saliva from their child at the time of consent. Mothers were instructed to collect on their child using a soft nontoxic sponge; they were instructed to place the sponge under the child’s tongue and swipe around his or her mouth and gums in order to soak up saliva for a total of 90 seconds. If they encountered resistance from their child, they were advised to try to break up the collection into intervals of 15 to 30 seconds. Participants were also emailed a link to an instructional video on how to collect from their child. Mothers collected saliva on themselves and on their child twice a day (immediately upon waking and again 30 minutes later) for four consecutive days (two non-workdays followed by two workdays). On each morning that saliva was collected, participants filled out questionnaires designed to assess compliance with the saliva samplings. Research assistants called participants the night before their first collections to remind them to begin the following morning. Participants were instructed to collect their first sample immediately upon awakening, right when they opened their eyes and before they got out of bed. At this time they were reminded not to eat, drink, or smoke before collecting their second sample; however, to increase hydration, they were asked to drink a glass of water 10 minutes before their second collection. Mothers also responded to questionnaires assessing demographic information. Families were compensated $100 after completion.
Measures
The psychobiology of stress
Mothers expressed saliva through a short straw into a small vial, and collected saliva from their child using an absorbent sponge (Salimetrics Children’s Swab). Mothers were instructed to swab under the child’s tongue and along the gum line for approximately 90 seconds. After collecting, mothers were told to immediately store the samples in the freezer. Samples were transported on ice to Purdue University and then stored frozen at −80 °C until assayed. To release the saliva from the child swabs and access the clear top-phase of the mothers’ samples, samples were brought to room temperature and centrifuged at 3,000 RPM for 15 minutes. All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test has a range of sensitivity from 0.007 to 1.8 g/dL, and average intra- and inter-assay coefficients of variation of less than 10% and 15%, respectively. All samples were assayed in duplicate and the average of the duplicates was used in all analyses. Cortisol levels were natural log transformed to correct for skewed distributions.
Saliva collection questionnaire
Mothers filled out a questionnaire designed to assess compliance with collection procedures on each saliva collection morning. Mothers were asked questions about the night before and the morning of each collection (e.g., “how many hours did you sleep,” “what time did you wake up”) and what time they collected each sample. Mothers answered these questions twice, once regarding themselves and once regarding their child. Mothers were also asked to provide a reason if they did not collect their child’s sample immediately upon awakening; additionally, they were asked to provide their child’s true wake-up time in order to deduct how much time had passed between the collection and actual wake time. Following Broderick and colleagues [36], a 15 minute “compliance window” was employed. First morning samples that were collected within 15 minutes of awakening, and second samples collected between 15 and 45 minutes post-awakening, were considered compliant. Non-compliant mother and child samples (n = 10) were not included in the analyses. Time between first and second collections ranged from 20 to 45 minutes.
Missing data
Out of the 752 samples collected, 11% of the mother samples and 18% of the child samples were missing. This resulted in 28 mothers and 13 children of the 47 dyads having cortisol values for all eight collection points. Three children and one mother were missing cortisol for every day. The high rate of missingness for the child was mainly due to insufficient sample volume to assay. Maternal and child cortisol outliers three standard deviations above and below the mean were removed (cortisol levels: n = 4 and CARs: n = 3).
Data analysis
Average cortisol levels are operationalized as the average of the awakening and 30 minutes post samples, while the cortisol awakening response is operationalized as the change from awakening cortisol levels to the cortisol levels at 30 minutes post-awakening. Awakening and 30 minutes post values were required to calculate cortisol levels and CARs. First, descriptive statistics of morning cortisol levels and CARs will be provided along with demographic characteristics and potential control variables associated with maternal and child cortisol levels and awakening responses. Using a series of mixed models [37], workday/non-workday differences in mother, child, and mother-child cortisol were examined. Models 1 – 3 were analyzed twice; once examining maternal cortisol, and once examining child cortisol.
Cortisol stability from non-work to workday
We first examined the predictive power of non-workday cortisol on workday cortisol, with the Level 1 outcome lnWorkCortij referring to the natural log of the average morning workday cortisol levels for person j on day i. It is a function of β0j, the person’s own intercept, and β1j, the person’s own slope, which is characterized by the association with non-workday cortisol levels (nonWorkCortij), and eij, the within-person residual. As a significant within subject covariate, WakeTime (β2j) was included in Level 1 equations, whereas between subject control variables (e.g., household income) were entered at Level 2 for all models with cortisol as the dependent variable. To model CAR, the Level 1 equation was similar to the levels model, apart from using the non-work and work CAR variables and covariates. The workday cortisol levels model is as follows:
-
(1)
Level 1: lnWorkCortij = β0j + β1j(nonWorkCortij) + β2j(WakeTimeij) + eij
Changes in cortisol across the morning
Next, we modeled the within-person change in cortisol from awakening to 30 minutes post-awakening. At Level 1, the outcome, lnCortij refers to the natural log of the average morning cortisol levels for person j on day i. It is a function of β0j, the person’s own intercept, and β1j, the person’s own slope, which is characterized by the association between whether the sample was collected at awakening or not (Awakeningij), wake time (β2j), and eij, the within-person residual. The Awakeningij variable is a dichotomous variable referencing the cortisol value either as Awakening (0) or 30 minute post (1). Between subject control variables were entered at Level 2. The cortisol levels model is as follows:
-
(2)
Level 1: lnCortij = β0j + β1j(Awakeningij) + β2j(WakeTimeij) + eij
Differences in workday and non-workday cortisol
Third, we modeled the within-person differences in workday/non-workday cortisol. At Level 1, the outcome, lnAvgCortij refers to the natural log of the average AM cortisol levels for person j on day i. It is a function of β0j, the person’s own intercept, and β1j, the person’s own slope, which is characterized by the association between whether the day was a workday or not (Workdayij), WakeTime (β2j), and eij, the within-person residual. For CAR, the modeling of the Level 1 equation was identical to the levels model, apart from the dependent variable and covariates. The Workdayij variable is a dichotomous variable referencing the cortisol value (levels or CAR) either as Non-work (0) or Workday (1). Between subject control variables were entered at Level 2. The cortisol levels model is as follows:
-
(3)
Level 1: lnAvgCortij = β0j + β1j(Workdayij) + β2j(WakeTimeij) + eij
Concordance of mother and child cortisol
Finally, we modeled the within-dyad differences in workday/non-workday cortisol concordance. At Level 1, the outcome, lnAvgChildCortij refers to the natural log of the average morning cortisol levels for child j on day i. It is a function of β0j, the child’s own intercept, and β1j, the child’s own slope, which is characterized by the association with maternal cortisol (AvgMomCortij), whether the day was a workday or not (Workdayij), the child’s WakeTtime (β2j), and eij, the within-person residual. In addition, a maternal cortisol by workday interaction (AvgMomCortij *Workdayij) was added to the model to determine the difference in mother-child cortisol attunement on non-work compared to workdays. For CAR, the modeling of the Level 1 equation was identical to the levels model, apart from the dependent variable and covariates as well as using maternal CAR as the independent variable. The concordance model for levels is as follows:
-
(4)
Level 1: lnAvgChildCortij = β0j + β1j(AvgMomCortij) + β2j(Workdayij) + β3j(ChildWakeTimeij) + β4j(AvgMomCortij * Workdayij) + eij
All modeling was carried out using Proc MIXED in SAS 9.2. Full information maximum likelihood estimation (MLE) was used for all models, which has been shown to produce unbiased parameter estimates and standard errors in models with missing data. MLE provides the correct likelihood for the missing parameters based on the distribution of the observed data and provides valid standard errors when calculated based on the observed information matrix [38–39].
Results
Preliminary and descriptive analyses
Mother and child person-specific (e.g., age, report of health status, household income, maternal marital status, maternal smoking status, medication use) and day-specific (e.g., number of hours slept, bed time, wake time, amount of time elapsed between awakening and the first sample, amount of time elapsed between the first and second samples) variables were examined as potential control variables for mother and child cortisol levels and awakening responses. Maternal wake time, β = −.19, p = .002, and household income, β = .18, p = .006, were related to maternal cortisol levels; earlier wake times and higher income were related to higher average cortisol levels. Similarly, child wake time was related to child cortisol levels, β = −.26, p = .01, with earlier wake times related to higher levels. Mother and child wake times were highly correlated, r = .79, p < 001; however, children woke on average 25 min (SD = 48 min) after their mothers on workdays and 12 min (SD = 58 min) after their mothers on non-workdays. No other potential control variables were related to maternal or child cortisol levels or CARs. Lastly, higher maternal morning cortisol levels were related to steeper maternal awakening responses, β = .17, p = .004. However, child cortisol levels were not related to their awakening responses, β = .01, ns.
Main analyses
Mother cortisol levels and awakening responses
As expected, higher maternal non-workday cortisol levels predicted higher maternal workday cortisol levels, β = .75, p = .01, and steeper maternal non-workday CARs were marginally related to steeper workday CARs, β = .20, p = .08. As reported elsewhere [blinded for review], maternal cortisol levels increased from waking (M = .36 ug/dL, SD = .19) to 30 min post (M = .42 ug/dL, SD = .23), controlling for wake time, β = .29, p < .001. Further, while mothers exhibited a significant increase in cortisol on both work, β = .53, p = .006, and non-workdays, β = .18, p = .05, mothers had steeper cortisol increases on workdays (M = .13 ug/dL, SD = .25) compared to non-workdays (M = .04 ug/dL, SD = .19), β = .53, p = .006. There was no workday/non-workday difference in cortisol levels, controlling for wake time, β = .12, ns.
Child cortisol levels and awakening responses
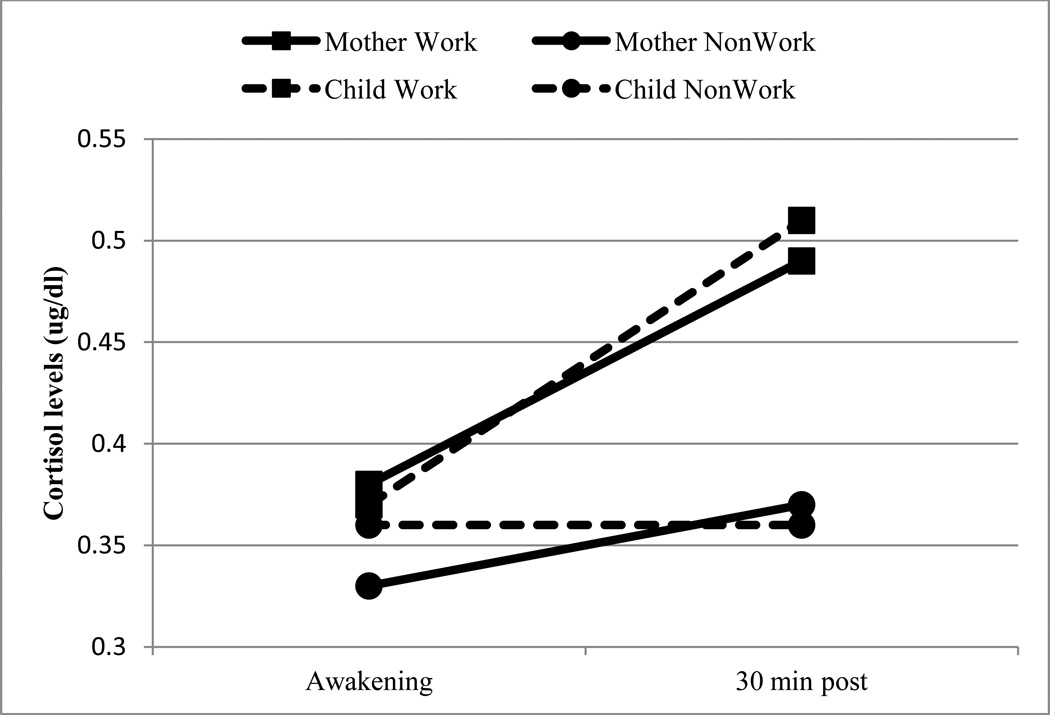
As expected, children’s non-workday cortisol levels predicted workday cortisol levels, β = 1.33, p = .003; however, non-workday CARs did not predict workday CARs, β = .01, ns. Overall, children did not show a change in cortisol levels from wake-up (M = .37 ug/dL, SD = .27) to 30 min post (M = .44 ug/dL, SD = .33), controlling for wake time, β = .11, ns. However, workday was related to children’s cortisol levels, β = .34, p = .01, and CARs, β = .24, p = .03, with higher levels and steeper CARs on workdays compared to non-workdays (Fig. 1). Follow-up analyses revealed cortisol increased significantly from waking to 30 min post on days children’s mothers went to work, β = .29, p = .001, but not on non-workdays.
Figure 1.
Mothers have higher levels and steeper CARs than children. Both mothers and children have significantly steeper CARs on workdays compared with non-workdays.
Mother-child cortisol levels and awakening responses
Mixed modeling was used to examine the relationships between mother and child cortosol levels and awakening responses across the four collection days. Overall, mothers had higher cortisol levels, β = .10, p = .05, and steeper CARs, β = .14, p = .02, than children (Fig. 1). β = .26, p = .03, and maternal CARs predicted child CARs, β = .18, p = .03. Workday did not moderate the relationship between mother and child CARs, β = −.15, ns; however, there was a significant difference in the relationship between mother and child cortisol levels on workdays compared to non-workdays, β = −.36, p = .05. Follow-up analyses revealed maternal cortisol levels only predicted child levels on non-workdays, β = .67, p = .004.
Discussion
The purpose of this study was to examine the relationship between maternal workday, and child morning cortisol and mother-child cortisol attunement in a sample of working mothers with children who attend out-of-home child care. Supporting past literature [40–41], we found intra-individual stability in working mothers’ morning physiology. Interestingly, less stability was seen in children’s morning physiological profiles. While children exhibited relative stability in average cortisol levels across days, children’s CARs were highly variable, with non-workday CARs not predictive of workday CARs. Further, children did not exhibit an increasing CAR on non-workdays, but did show an increase in cortisol from awakening to 30 minutes post on workdays. In addition, maternal cortisol levels predicted child levels, and maternal CARs predicted child CARs. Finally, workday moderated the mother-child cortisol level relationship revealing mother-child cortisol concordance only on non-workdays. This study highlights the importance of understanding families’ social contexts in studies of children’s morning cortisol.
Previous research highlights the importance of workday as a significant contextual factor influencing adult physiology. Specifically, a workday/non-workday difference in morning cortisol is normative among working adults [4], who report greater workload on workdays [42]. However, this study illustrates workday may also be important for the physiological functioning of young children of working parents. In fact, it appears that when taking maternal workday into consideration, children also exhibit higher cortisol levels and steeper CARs on workdays compared to non-workdays. While the current study was not able to test specific mechanisms, children’s physiological profiles could be a reflection of changes in maternal and/or child behavior on work compared to non-workdays.
Adult morning physiology tends to be predicted by both momentary and anticipatory emotions [43]. However, given the egocentric nature of preschoolers [44], children’s morning physiology may be a stronger reflection of the immediate context as opposed to anticipation of the upcoming daycare day. As such, children’s heightened workday CARs suggest the interactions and routines surrounding workday mornings may be more physiologically arousing than those on non-workday mornings. In qualitative interviews, working mothers report rushed workday mornings with slight deviations from everyday routines being viewed as a source of stress [45]. Children’s greater physiological arousal could be related to changes in maternal behavior [46] as mothers must focus on competing tasks [47] (i.e., preparing themselves for work and their child for child care). Likewise, children’s differential physiology could be associated with changes in child behavior. Workday mornings may be a time of greater child physical activity as children ready themselves for the day. Physiological activation may be heightened to support this level of arousal [48] as well as to meet the expectations of compliance [49] surrounding morning routines. In contrast, children may have fewer physical and emotional demands in the first 30 minutes of a non-workday morning when they are less likely to leave the house first thing in the morning on a set schedule.
Thus, workday mornings in families with children attending out of home care may present a combination of experiences (e.g., changes in maternal and child behavior) with the potential to increase child physiological activation. However, workdays are not the only days/mornings where these changes coincide, and child cortisol may also increase in other, but similar family contexts (e.g., leaving the house by a specific time for a non-work/daycare event). Importantly, because workday cortisol was only assessed on days both members of the dyad were leaving the house, it is not clear if both of these experiences (maternal work and out of home care) are necessary to raise child cortisol above non-work day cortisol. It could be that the child’s cortisol would still be heightened on days they stay home but their mothers left for work, or days they go to child care but their mothers stay home. That said, it is probably not the event itself that is related to cortisol in these young children, but the behaviors and interactions surrounding these events. Therefore, building off of the ‘risky family model,’ we hypothesize that any situations where mothers use harsh parenting, have greater expectations for compliance, or when children are meeting these emotional or physical demands, child morning cortisol will be heightened [50]. Additional studies are necessary to parse out the exact mechanisms and contexts responsible for the physiological changes found in the present analyses.
As expected, this study confirms a positive association between maternal and child cortisol levels and CARs. This association can partially be explained by shared genetic factors, considering heritability estimates of CAR are between 32 – 48% [41, 51]. Undoubtedly, however, contextual factors are also important in the degree of concordance between mother and child physiology [7]. The current analyses found a difference in the coordination of mother and child cortisol levels on work vs. non-workdays, with greater similarity between mother and child cortisol levels on non-work compared to workdays. Importantly, there was no relationship between maternal and child cortisol levels on workday mornings. This finding highlights the variability in mother-child physiological attunement, showing that attuned cortisol levels are not a sole function of the relationship, but a function of the relationship in context.
Previous studies have suggested that maternal sensitivity facilitates mother-child adrenocortical coordination [30]; however, other studies have found harsh and punitive parenting [7] and situations marked by challenge [29] or negative affect [28] to increase cortisol attunement within a mother-child dyad. These findings suggest that both positive and negative relationship dynamics have the potential to engender concordance, and thus concordance may not be solely indicative of the quality of the relationship. However, it could be that attuned cortisol is indicative of, for better or worse, engaged interactions and shared emotions [52]. Although daily variation in mother-child interactions on work and non-workdays were not assessed, it has been suggested that adults may socially withdraw from family interactions in an attempt to cope with the demands of work stress [53]. In particular, independent observers rate mothers as less physically, emotionally, and cognitively engaged with their children on high stress workdays than on low stress workdays [26]. While the study by Repetti and Wood [53] focused on evening reunions, the psychological anticipatory stress [54] of workday mornings may encourage similar psychological withdrawal from mothers, thereby uncoupling mother-child cortisol levels. Thus, workday mornings may present a time when mothers and children physically share the same space and activities, but the dyad experiences some emotional and physiological disengagement. Interestingly, different processes seem to be involved with the coordination of mother and child CAR, which was not moderated by workday. This finding perhaps suggests that the attunement of CARs is less susceptible to environmental influences.
Family interactions and parental behavior are dynamic and dependent on the emotional and physical contexts in which they are embedded [55–56]. Given the past emphasis on maternal behavior as a main driver of mother-child cortisol attunement [30, 35], it is likely that attunement fluctuates with changes in mother-child interactions. Indeed, the current analyses corroborate past findings that mother-child cortisol attunement is not stable [28], and fluctuations may be related to changes in family routines that go along with maternal work schedules. However, our findings are most likely not unique to the workday context. While this study does not elucidate the mechanisms at play, based off of the work by Feldman and colleagues [57], we hypothesize that emotional engagement may be important for physiological coordination. Thus, future researchers should examine emotional withdrawal in this context, as well as other family contexts in which it is likely to occur, to determine the effect it has on cortisol attunement.
Future directions/Limitations
Assessing morning cortisol levels in young children poses unique challenges for researchers because of children’s inability to collect their own saliva. This potentially doubles the chances of noncompliance due to the necessity for both caregiver and child to adhere to the protocol (in our study, 18% of children’s cortisol was missing compared to mothers’ 11%). Noncompliance/missingness could have biased results by reducing the power necessary to detect a smaller magnitude CAR on non-workdays. However, maximum likelihood estimation is a robust method for accounting for missing data and is known to produce unbiased estimates of standard errors even with moderate amounts of missingness [58]; thus, it is unlikely that the missingness in the current sample affected the estimates produced.
Not adhering to the collection protocol jeopardizes the ability to accurately capture the awakening response, and has been related to higher awakening cortisol levels [59] and flatter CARs [60]. Therefore it could be that differences in morning structure and routine on work compared to non-workdays affected compliance, biasing the results. On workday mornings, mothers may be more likely to wake up before their child (or wake up their child) to get to work on time, thus ensuring child samples are collected upon awakening. In contrast, on non-workday mornings, children may wake before their mothers, despite maternal report of waking before her child. It should be noted, however, that mothers do not self-report lower compliance on non-workdays, and follow-up analyses do not reveal a difference in awakening cortisol on work compared to non-workdays in the children. Rather, the difference in workday/non-workday cortisol levels and CARs in the children seems to be driven by their 30 minute post samples. Regardless, researchers should consider incorporating multiple methods to assess sleep and collection time when collecting morning cortisol on children [18].
Lastly, it is important to note that weekday and weekend mornings could differ for families regardless of whether the mother is employed. The activity of weekday mornings may be driven by an older sibling’s school schedule, preschool attendance, a father’s work schedule, or different combinations of these factors on different days. Families must coordinate multiple individuals’ schedules and some families may be more routine-oriented in this endeavor than others [61]. While the current study only examined mothers employed outside of the home and their children cared for in out of home care, and thus did not compare different family configurations (e.g., families without a working mother), future studies should examine the relationship between day-to-day physiology and family routines in multiple family types. Further, the data presented are preliminary in that while daily physiology was collected, researchers should strive to collect more detailed information on daily variation in morning routines, behavior, and affect, in addition to physiological markers.
Conclusions
Family systems scholars have long recognized the inter-related nature of family members and the propensity for family members to impact each other’s thoughts, feelings, and actions [62]. Similarly, biosocial researchers recognize the potential for social context and interpersonal interactions to exert a profound influence over physiological arousal [9]. Importantly, these dynamic biobehavioral relationships among parents and children have the potential to determine the set-point for life-long physiological reactivity [63] and initiate differential life course trajectories [64]. As such, attuned physiological processes may provide another mechanism by which early rearing environments affect later mental and physical health [65, 31].
Acknowledgements
We would like to thank the many families who took time out of their busy schedules to participate in this project. We would also like to thank Rebecca Jarvis-Caruthers, Umadevi Senguttuvan, Elizabeth Langston, and Shilpa Parakh, whose invaluable help made this study possible.
Role of Funding Source
The first author was supported by a grant from the National Institute of Child Health and Human Development (R21 HD066269-01A1). The National Institute of Child Health and Human Development had no further involvement in: the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
There are no conflicts of interest related to this paper.
References
- 1.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Hibel LC, Mercado E, Trumbell JM. Parenting stressors and morning cortisol in a sample of working mothers. J Fam Psychol. 2012;26(5):738–746. doi: 10.1037/a0029340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on workdays and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29(4):516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 6.Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: age differences and behavioral correlates. Child Dev. 2003;74(4):1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- 7.Hibel LC, Granger DA, Blair C, Cox MJ. Intimate partner violence moderates the association between mother-infant adrenocortical activity across an emotion challenge. J Fam Psychol. 2009;23(5):615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- 8.Bright MA, Granger DA, Frick JE. Do infants show a cortisol awakening response? Dev Psychobiol. 2012;54(7):736–743. doi: 10.1002/dev.20617. [DOI] [PubMed] [Google Scholar]
- 9.Booth A, Carver K, Granger DA. Biosocial perspectives on the family. J Marriage Fam. 2000;62(4):1018–1034. [Google Scholar]
- 10.Nelson RJ. An introduction to behavioral endocrinology. Sunderland (MA): Sinauer Associates; 2011. [Google Scholar]
- 11.Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neurosci Biobehav Rev. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonini SRR, Jorge SM, Moreira AC. The emergence of salivary cortisol circadian rhythm and its relationship to sleep activity in preterm infants. Clin Endocrinol. 2000;52(4):423–426. [PubMed] [Google Scholar]
- 15.de Weerth C, Zijil R, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Hum Dev. 2003;73(1–2):39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 16.Tegethoff M, Knierzinger N, Meyer AH, Meinlschmidt G. Cortisol awakening response in infants during the first six postnatal months and its relation to birth outcome. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.08.002. In press. [DOI] [PubMed] [Google Scholar]
- 17.Stalder T, Bäumler D, Miller R, Alexander N, Kliegel M, Kirschbaum C. The cortisol awakening response in infants: ontogeny and associations with development-related variables. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.07.015. In press. [DOI] [PubMed] [Google Scholar]
- 18.Gribbin CE, Watamura SE, Cairns A, Harsh JR, LeBourgeois MK. The cortisol awakening response (CAR) in 2-to 4-year-old children: effects of acute nighttime sleep restriction, wake time, and daytime napping. Dev Psychobiol. 2011;54(4):412–422. doi: 10.1002/dev.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam EK, Klimes-Dougan B, Gunnar MR. Social regulation of the adrenocortical response to stress in infants, children, and adolescents. In: Coch D, Dawson G, Fischer KW, editors. Human behavior, learning, and the developing brain: Atypical development. New York (NY): Guilford; 2007. pp. 264–304. [Google Scholar]
- 20.Gunnar MR, Quevedo KM. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 21.Saxbe DE. A field (researcher's) guide to cortisol: tracking HPA axis functioning in everyday life. Health Psychol Rev. 2008;2(2):163–190. [Google Scholar]
- 22.Pendry P, Adam EK. Associations between parents' marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev. 2007;31(3):218–231. [Google Scholar]
- 23.Slatcher RB, Robles TF. Preschoolers' everyday conflict at home and diurnal cortisol patterns. Health Psychol. 2012;31(6):834–838. doi: 10.1037/a0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronfenbrenner U. Ecology of the family as a context for human development: research perspectives. Dev Psychol. 1986;22(6):723–742. [Google Scholar]
- 25.Hochschild AR, Machung A. The second shift: working parents and the revolution at home. 2nd edition. New York: Penguin Books; 2003. [Google Scholar]
- 26.Repetti RL, Wood J. Effects of daily stress at work on mothers' interactions with preschoolers. J Fam Psychol. 1997;11(1):90–108. (1997). [Google Scholar]
- 27.Slatcher RB, Robles TF, Repetti RL, Fellows MD. Momentary work worries, marital disclosure, and salivary cortisol among parents of young children. Psychosom Med. 2010;72(9):887–896. doi: 10.1097/PSY.0b013e3181f60fcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: within-family cortisol associations and moderators. J Fam Psychol. 2009;23(6):882–894. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, Shirtcliff EA. Adrenocortical attunement in mother-child dyads: importance of situational and behavioral characteristics. Biol Psychol. 2011;88(1):104–111. doi: 10.1016/j.biopsycho.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27(6):731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- 31.Schore AN. Attachment and the regulation of the right brain. Attach Hum Dev. 2000;2(1):23–47. doi: 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- 32.Women in the labor force: a databook–2013 [Internet] Washington: Department of Labor (US); 2013. Feb, [cited 2013 March 26]. Bureau of Labor Statistics; p. 104. Report No. 1040. Available from: http://www.bls.gov/cps/wlf-databook–2012.pdf. [Google Scholar]
- 33.America’s children: key national indicators of well-being, 2011 [Internet] Washington: US Government Printing Office; 2011. Jul, [cited 2012 Nov 8]. Federal Interagency Forum on Child and Family Statistics (US) p. 223. Available from: http://www.childstats.gov/pdf/ac2011/ac_11.pdf. [Google Scholar]
- 34.Posada G, Jacobs A, Carbonell OA, Alzate G, Bustamante MR, Arenas A. Maternal care and attachment security in ordinary and emergency contexts. Dev Psychol. 1999;35(6):1379–1388. doi: 10.1037//0012-1649.35.6.1379. [DOI] [PubMed] [Google Scholar]
- 35.van Bakel HJA, Riksen-Walraven JM. Adrenocortical and behavioral attunement in parents with 1-year-old infants. Dev Psychobiol. 2008;50(2):196–201. doi: 10.1002/dev.20281. [DOI] [PubMed] [Google Scholar]
- 36.Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29(5):636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 37.Bryk AS, Raudenbush SW. Hierarchical linear models in social and behavioral research: applications and data analysis methods. 1st edition. Newbury Park, CA: Sage; 1992. [Google Scholar]
- 38.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 39.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 40.Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 41.Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- 42.Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med. 2003;65(1):92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 43.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience– cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U.S.A. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piaget J. Science of education and the psychology of the child. Oxford: Orion; 1970. [Google Scholar]
- 45.Maume DJ, Sebastian RA, Bardo AR. Gender, work-family responsibilities, and sleep. Gend Soc. 2010;24(6):746–768. [Google Scholar]
- 46.Hasting PD, Ruttle PL, Serbin LA, Mills RSL, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: relations with parenting, temperament, and psychopathology. Dev Psychobiol. 2011;53(7):694–710. doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- 47.Smith PB, Pederson DR. Maternal sensitivity and patterns of infant-mother attachment. Child Dev. 1988;59(4):1097–1101. doi: 10.1111/j.1467-8624.1988.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 48.de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12(2):95–164. [Google Scholar]
- 49.Stifter CA, Spinrad T, Braungart-Rieker J. Toward a developmental model of child compliance: the role of emotion regulation in infancy. Child Dev. 1999;70(1):21–32. doi: 10.1111/1467-8624.00003. [DOI] [PubMed] [Google Scholar]
- 50.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128(2):330–366. [PubMed] [Google Scholar]
- 51.Kupper N, de Geus EJC, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30(9):857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Saxbe D, Repetti RL. For better of worse? Coregulation of couples’ cortisol levels and mood states. J Pers Soc Psychol. 2010;98(1):92–103. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- 53.Repetti RL. Effects of daily workload on subsequent behavior during marital interaction: the roles of social withdrawal and spouse support. J Pers Soc Psychol. 1989;57(4):651–659. doi: 10.1037//0022-3514.57.4.651. [DOI] [PubMed] [Google Scholar]
- 54.Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30(6):599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Belsky J. The determinants of parenting: a process model. Child Dev. 1984;55(1):83–96. doi: 10.1111/j.1467-8624.1984.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 56.Posada G, Kaloustian G, Richmond MK, Moreno AJ. Maternal secure base support and preschoolers’ secure base behavior in natural environments. Attach Hum Dev. 2007;9(4):393–411. doi: 10.1080/14616730701712316. [DOI] [PubMed] [Google Scholar]
- 57.Feldman R, Greenbaum CW, Yirmiya N. Mother–infant affect synchrony as an antecedent of the emergence of self-control. Dev Psychol. 1999;35(1):223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- 58.Singer JD, Willett JB. Applied longitudinal data analysis: methods for studying change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 59.Okun ML, Krafty RT, Buysse DJ, Monk TH, Reynolds Iii CF, Begley A, Hall M. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2010;35(3):460–468. doi: 10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65(2):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- 61.Daly K. Time, gender, and the negotiation of family schedules. Symbolic Interaction. 2002;25(3):323–342. [Google Scholar]
- 62.Cox MJ, Paley B. Families as systems. Annu Rev Psychol. 1997;48(1):243–267. doi: 10.1146/annurev.psych.48.1.243. [DOI] [PubMed] [Google Scholar]
- 63.Zhang TY, Meaney MJ. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol. 2010;61(1):439–466. doi: 10.1146/annurev.psych.60.110707.163625. [DOI] [PubMed] [Google Scholar]
- 64.Belsky J. The development of human reproductive strategies: progress and prospects. Curr Dir Psychol Sci. 2012;21(5):310–316. [Google Scholar]
- 65.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]