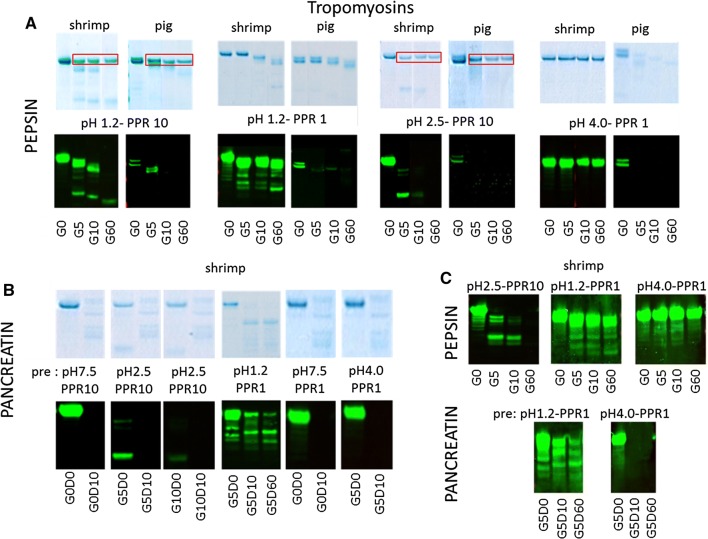
Fig. 4.
Selected SDS-PAGE and immunoblot samples are shown for both tropomyosins, from shrimp (Pen a 1) and pig. A At pH 1.2 and 2.5 judged by SDS-PAGE no very clear difference in resistance is apparent, although for shrimp tropomyosin the presence of breakdown peptides at PPR 10 appears to be more prominent. Pepsin bands visible at PPR 10 are boxed in red. At PPR 1 resistance of both tropomyosins appears quite high. Immunoblot analyses of gastric samples exposed to pH 1.2 and 2.5 confirm higher stability of shrimp tropomyosin but it cannot be excluded that this is rather a result of different affinities of rabbit antisera. The most convincing difference between allergen and non-allergen is observed at pH 4.0, with shrimp tropomyosin being totally unaffected and pig being readily digested. B Shrimp tropomyosin is highly susceptible to pancreatin except when having been pre-exposed to pH 1.2. C IgE immunoblotting confirms pepsin and pancreatin resistance characteristics observed wit rabbit IgG