Abstract.
Plasmodium falciparum gametocytes develop over 9–12 days while sequestered in deep tissues. On emergence into the bloodstream, they circulate for varied amounts of time during which certain host factors might influence their further development. We aimed to evaluate the potential association of patient clinical parameters with gametocyte development and carriage via in vivo methods. Seventy-two patients were enrolled from three hospitals in the Volta region of Ghana in 2016. Clinical parameters were documented for all patients, and gametocyte prevalence by microscopy was estimated at 12.5%. By measuring RNA transcripts representing two distinct gametocyte developmental stages using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR), we obtained a more precise estimate of gametocyte carriage while also inferring gametocyte maturation. Fifty-three percent of the study participants harbored parasites expressing transcripts of the immature gametocyte-specific gene (PF3D7_1477700), whereas 36% harbored PF3D7_1438800 RNA-positive parasites, which is enriched in mid and mature gametocytes, suggesting the presence of more immature stages. Linear logistic regression showed that patients older than 5 years but less than 16 years were more likely to carry gametocytes expressing both PF3D7_1477700 and PF3D7_1438800 compared with younger participants, and gametocytemia was more likely in mildly anemic individuals compared with those with severe/moderate anemia. These data provide further evidence that a greater number of malaria patients harbor gametocytes than typically estimated by microscopy and suggest a possible association between age, fever, anemia, and gametocytemia.
INTRODUCTION
Malaria is one of the most important infectious parasitic diseases and was responsible for approximately 445,000 deaths globally from more than 216 million clinical cases in 2016.1 Among the different malaria parasites, P. falciparum is the most virulent and accounts for most malaria-associated mortality and morbidity in sub-Saharan Africa. In the Volta region of Ghana, P. falciparum is the main cause of malaria, and it is the responsible agent in 98% of hospitalized malaria patients.2 The parasites are transmitted through the bite of infected anopheline mosquitoes, with the pathology resulting from the asexual replication of parasites within circulating red blood cells, leading to anemia, disrupted blood flow, and inflammation that can be lethal if untreated. The transmission of malaria from humans to mosquitoes is mediated by specialized sexual stages called gametocytes, which are derived from asexual parasites. Gametocytes of P. falciparum have a prolonged development of 9–12 days, maturing through five distinct morphological and metabolic stages. In vivo, stages I–IV are sequestered in the spleen and the hematopoietic system and the extravascular space of the bone marrow3–6 whereas mature stage V gametocytes are present in peripheral circulation. Mature gametocytes circulate in the bloodstream for a varied number of days ranging 1.3–22.2 days, during which period they are available to biting mosquitoes.7–10 In vitro, gametocytes can persist for about 16–32 days.9
A number of host factors have been linked to gametocyte development and appearance in the presence of treatment. Age, hematocrit, hemoglobin genotype, fever, and duration of symptoms have all been associated with gametocytemia in treated patients.11–13 In addition, drug treatment and host immune responses have been linked with different levels of gametocytemia.14–16 In in vitro experiments, specific parasite strains have been associated with different levels of gametocyte production and the serum used in gametocyte cultivation, suggesting the involvement of parasite genetic factors and host immune factors.17 In addition, microvesicles have been shown to mediate communication between malaria parasites, and the abundance of these vesicles correlated with high peripheral blood parasitemia18 and stimulation of gametocytes in vitro.19,20 Very recently, levels of the host serum factor lysophosphatidylcholine were shown to influence rates of sexual differentiation,21 providing additional evidence for the influence of host factors on parasite development.
The involvement of certain parasite genes in gametocyte commitment and production has been demonstrated. Notably, the expression of the P. falciparum gametocyte development 1 protein has been shown to be critical for gametocyte production. Available evidence localizes this protein to the nucleus, where it might be involved in regulating gametocyte production pathways.22,23 In addition, the transcription factor AP2-G has been identified as a master regulator for sexual development, and its expression is controlled epigenetically through the incorporation of the silencing histone mark, histone 3 lysine 9 trimethylation (H3K9me3), at the ap2-g locus.24,25
Although both the parasite and host factors are involved in regulating sexual differentiation and development, it is not known how these factors directly affect gametocyte production in vivo as most human studies have been in treated individuals. It is known that a variable percentage of asexual parasites develop into gametocytes, but how this is determined and regulated in vivo is not clearly understood, as early gametocyte markers have mainly been deployed in studies of cultured P. falciparum.9,10,22 In this study, we measured transcripts from two different gametocyte-specific genes as indicators of early and late stages of sexual development, respectively, and related gene expression to patient clinical parameters. As previously described,26 these parasite stage-specific genes were selected based on their stage specificity and transcript abundance. Genes representing three developmental stages of P. falciparum were used: ring stage asexual trophozoites (TR, PF3D7_0501300), early-mid developing gametocytes (DG, PF3D7_1477700), and mid-late maturing gametocytes (MG, PF3D7_1438800) to enable the assessment of the development of gametocytes in malaria patients. The ring stage marker, TR, is a skeleton-binding protein associated with the infected erythrocyte skeleton that is highly expressed in ring stage TR compared with other life cycle stages. The early-mid gametocyte marker, DG, is an exported protein with a Plasmodium helical interspersed subtelomeric A domain whereas the mid-early gametocyte marker, MG, is a conserved protein with an OST-HTH–associated domain. The functions of DG and MG are unknown, but DG is thought to be enriched specifically in asexual stages committed toward the sexual pathway22 whereas MG is highly expressed in gametocytes stages III–V.27 All three genes are intron-containing markers that enables the avoidance of genomic DNA amplification, by contrast to the established immature and mature gametocyte markers Pfs16 and Pfs25. In addition, the frequency of gametocytemia in infections carrying gene variants associated with quinolone- and antifolate- drug resistance was assessed. The findings will inform the design of new strategies to more accurately dissect gametocyte production in patients and allow a more detailed analysis of the mechanisms involved in gametocyte maturation, which could lead to the development of transmission-blocking interventions.
MATERIALS AND METHODS
Ethics statement.
The study obtained ethical clearance from the Ghana Health Service Ethics Review Committee in Accra (ID No: GHS-ERC 03/05/15) and the internal review board at the Weill Cornell Medical College in New York (IRB#1508016447). In addition, written informed consent and accent, in the case of children between 10 and 14 years, were obtained from individuals/parents/guardians before enrollment into the study. The study procedures, risks, and benefits were explained to all study participants and caregivers. Positive asexual parasites and gametocyte control samples for reverse transcriptase quantitative polymerase chain reactions were obtained from culture-adapted parasite isolates from among the study samples.
Study area.
This was a hospital-based study conducted between June and July 2016 in Ho in the Volta region, located in the southeastern part of Ghana where it borders Togo. The study area, environment, and malaria situation have been previously described.2 Plasmodium falciparum is responsible for almost all clinical malaria in this area. We had previously found that Plasmodium malariae was PCR-detectable in 2% of infections (5/211) in this area, and that all but one were coinfections with P. falciparum.2 To date, no Plasmodium ovale has been detected. The transmission of malaria in this area is variable but usually peaks one month after the start of each of the two rainy seasons, which take place from April to July and from September to November. The entomological inoculation rate for a nearby town, Hohoe, was estimated to be 65 infectious bites per person per year.28
Patients and sample collection.
Patients of all ages attending outpatient clinics at the Ho Polyclinic, the Ho Municipal Hospital, and Volta Regional Hospital were screened for malaria by rapid diagnostic tests (RDT) (Standard Diagnostics, Korea) and microscopy using finger-prick blood. Rapid diagnostic tests or microscopy-positive individuals, who gave their consent, were enrolled into the study to investigate the human host and parasite factors modulating the gametocyte development. Up to 5 mL of venous blood was collected in heparinized tubes from all participants for slide preparation, RNA preservation in Trizol (Invitrogen, Carlsbad, CA), parasite culture, and plasma separation. An additional blood sample of approximately 0.5 mL was collected in an ethylenediaminetetraacetic acid tube for the determination of hematological parameters using a Sysmex hematology analyzer (Sysmex America, Irvine, CA). Briefly, plasma from venous blood was separated by centrifuging at 2,000 rpm for 10 minutes. The resulting parasite material was used for cryopreservation, parasite culture, and parasite nucleic acid (RNA) preservation in Trizol and blood smears. Once samples were collected, participants were assisted to follow hospital procedures for disease management with no subsequent follow-up by us. Patients who had other illnesses in addition to malaria or had taken antimalarials in the preceding 14 days were excluded from the study.
Assessment of asexual parasitemia and gametocytemia.
Microscopy.
Thin and thick smears were stained with 10% Giemsa for microscopic diagnosis and subsequent determination of parasitemia. Thin blood smears were fixed with methanol before Giemsa staining. Estimation of parasite densities was carried out by counting asexual parasites against 200 leukocytes whereas counting gametocytes against 500 leukocytes. Both parasite counts were converted to parasites per microliter by assuming a standard leukocyte count of 8,000 leukocytes in one microliter of blood, as already described.29,30 The slides were declared negative if no asexual parasites were seen after the examination of 100 microscopic fields.
Reverse transcriptase quantitative PCR.
RNA extraction, DNase digests, and reverse transcription.
Fifty microliters of whole blood samples obtained from patients were stored in 500 µL of Trizol at −80°C until RNA extraction. Total RNA from the samples was first extracted using the phenol–chloroform method, as previously described,31 and then purified using the AllPrep DNA/RNA Mini Kit (QIAGEN, Hilden, Germany) following the instructions from the manufacturer. The resulting RNA was treated with DNase (Invitrogen) and stored at −80°C. The SuperScript III First Strand Synthesis kit from Invitrogen was then used to synthesize the first-strand cDNA.
Quantitative PCR assay.
Amplification and quantification of stage-specific gene transcripts (rings PF3D7_0501300, early-mid gametocytes PF3D7_1477700, and mid-late gametocytes PF3D7_1438800)26 were performed using the QuantStudio5 (Applied Biosystems, Foster City, CA). Previously reported primer sets that target genes representing the aforementioned stages were used.26 All reactions were run in a total volume of 10 µL containing 1X PerfeCTa SYBR Green SuperMix, 0.5 µM of each of the forward and the reverse primers, and 2 µL of the target cDNA. The thermal profile for the reactions included 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The specificities of the resulting products were analyzed using the dissociation curve and experiments with nonspecific products were repeated. All samples were run in triplicate and the mean Ct values were determined. Parasite-stage positivity was based on the absence or presence of the stage-specific RNA transcripts determined by the Ct values ≤ 35. A positive control RNA sample (GDVRH008) was obtained from a culture-adapted isolate of known asexual and gametocyte stage composition determined by microscopy. The control isolate, GDVRH008, was chosen because the patient was gametocyte-positive by microscopy and was readily inducible to produce gametocytes in vitro. This sample was positive also for all three stages of the parasite by RT-qPCR and at presentation had a gametocyte density of 16 parasites per microliter whereas the asexual parasite density was 26,160 parasites per microliter by microscopy.
Detection of single nucleotide polymorphisms (SNPs) in patient isolates.
Genomic DNA extraction, nested PCR, and restriction enzyme digestion.
The presence of antimalarial drug–associated SNPs in the clinical isolates was determined using PCR–restriction fragment length polymorphism. Briefly, DNA was extracted from all the samples using the AllPrep® DNA/RNA Mini Kit (QIAGEN) following the manufacturer’s instructions and then stored at –20°C. Both the outer and the nested PCRs were performed to amplify genomic regions that flank point mutations in the P. falciparum chloroquine resistance transporter gene (pfcrt) at position K76T; P. falciparum multidrug resistance gene one (pfmdr1) at positions N86Y and Y184F; P. falciparum dihydrofolate reductase gene (pfdhfr) at positions N51I, C59R, and S108N; and P. falciparum dihydropteroate synthetase (pfdhps) gene at positions A437G and K540E that have been implicated in antimalarial drug resistance. All reactions were performed in a total volume of 25 µL containing 1X of Maxima Hot Start Green PCR Master Mix (Thermo Scientific, Waltham, MA) and 200 nM of each of the forward and reverse primers. A volume of 0.5 µL of the initial PCR product was used as template DNA in the nested PCR and, 5 µL of the resulting product was separated on an ethidium bromide–stained 2% agarose gel before restriction digestion. PCR primer sets and cycling conditions for pfcrt and pfmdr1 were performed as previously reported32 whereas primers and conditions for pfdhfr and pfdhps also followed procedures already established.33 Using SNP-specific endonucleases (obtained from Thermo Scientific), 5 µL of the nested PCR product for each of the four genes was digested in a final volume of 15 µL. The restriction digest products were separated on an ethidium bromide–stained 2% agarose gel and the resulting gel was processed using the Amersham Imager 600 (General Electric, Boston, MA). Laboratory-adapted strains of P. falciparum of known genotypes were used as controls for the wild-type and the mutant alleles.
Data analysis.
Descriptive statistics was calculated using Stata version 14.2 (College Station, TX) and Microsoft Excel for determining means, percentages, standard deviation, and plotting of graphs. Age and hemoglobin levels of patients were categorized into four groups each (< 5, 5–9, 10–16, and > 16 years; and severe (< 8 g/dL), moderate (8–11 g/dL), mild (11–13 g/dL), and non-anemic (> 13 g/dL, respectively). For the purpose of logistic regression analysis, the severe anemia and moderate anemia groups were combined. These could therefore be considered as continuous or categorical variables depending on the analysis. The association between two continuous variables was assessed using Spearman’s rank correlation coefficient, whereas continuous and binary variables were assessed using Wilcoxon rank sum test. The linear logistic regression model was used to determine the association between age, hemoglobin, fever, and gametocyte carriage (MG).
RESULTS
Gametocyte carriage by microscopy.
A total of 72 (Volta Region Hospital = 10, Ho Polyclinic = 16, and Ho Municipal Hospital = 46) RDT-positive individuals were enrolled and included during the 2-month study period (Table 1). Fever, temperature > 37.5°C, was recorded in 65% of all participants examined. Asexual parasite prevalence by microscopy was 91.67% (66/72) (Table 1). Gametocyte prevalence by microscopy was 12.5% (9/72) (Table 1). The study participants were somewhat evenly distributed among the different age categories, and all gametocyte carriers were either moderately or mildly anemic. The geometric mean asexual parasite density by microscopy was 8,037.7 (95% confidence intervals: 5,075.9–12,727.8) parasites/µL whereas the geometric mean gametocyte density was 37.8 (95% CI: 24.7–58.0) parasites/µL (Table 1).
Table 1.
Characteristics of patients at enrollment were as follows: Anemia was defined as severe (< 8 g/dL), moderate (8–11 g/dL), mild (11–13 g/dL), and non-anemic (> 13 g/dL)
| Parameter | Value |
|---|---|
| No | 72 |
| Female: male | 41:31 |
| Age in years, median (range) | 13 (1–76) |
| Axillary temperature, median in °C (range) | 37.3 (36–40.6) |
| Fever, % (n/N) | 65 (47/72) |
| Hemoglobin level, median in g/dL (range) | 11.4 (3.4–16.2) |
| Microscopy | |
| Asexual parasite prevalence, % (n/N) | 91.67 (66/72) |
| Geometric mean asexual parasite density/μL (95% CI) | 8,037.7 (5,075.9–12,727.8) |
| Gametocyte prevalence, % (n/N) | 12.5 (9/72) |
| Geometric mean gametocyte density/μL (95% CI) | 37.8 (24.7–58.0) |
| Reverse transcriptase quantitative PCR | |
| PF3D7_0501300 RNA-positive ring stage prevalence, % (n/N) | 100 (72/72) |
| PF3D7_1477700 RNA-positive early-mid gametocyte prevalence, % (n/N) | 52.8 (38/72) |
| PF3D7_1438800 RNA-positive mid-late gametocyte prevalence % (n/N) | 36.1 (26/72) |
Fever was defined as temperature > 37.5°C.
Gametocyte carriage by RT-qPCR.
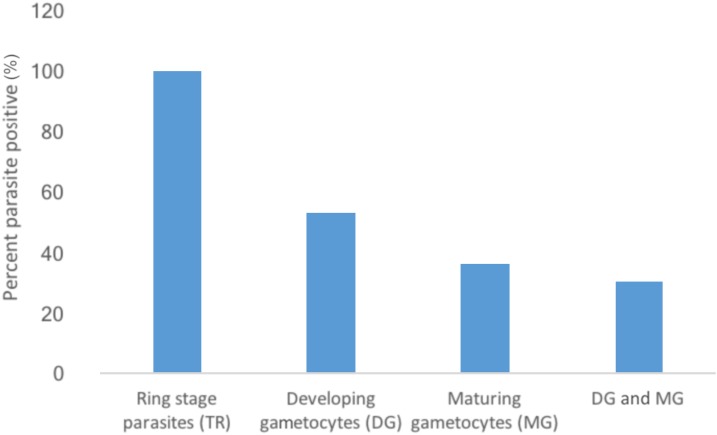
Parasite stage-specific RT-qPCR was used to determine the presence of TR (PF3D7_0501300), DG (PF3D7_1477700), and MG (PF3D7_1438800) transcripts in each infection. Transcripts of the TR gene26 were present in all individuals, including the 6 RDT-positive individuals who were negative for parasites by microscopy. The DG transcript was detected in 53% of individuals, whereas the MG transcript was detected in 36% (Figure 1). All nine individuals with detectable gametocytes by microscopy were positive for MG transcripts by RT-qPCR assay giving us a 100% specificity for microscopy but a significantly lower sensitivity than for RT-qPCR. Interestingly, four of the nine individuals with microscopically detectable gametocytes had no RT-qPCR signal for DG transcripts. The participants who were positive for either DG, MG or both transcripts (22/72) displayed mild or moderate anemia (32/38) but were evenly distributed between male and female patients (Table 2). The presence of the DG transcript is unexpected as DG are not found in the peripheral circulation and so should not have been captured in finger-prick sampling. However, the DG and MG transcripts have been previously characterized during gametocyte maturation in vitro,9,34 and it is unknown whether circulating sexually committed TR may also express the DG transcript in vivo.
Figure 1.
Proportion of patients with PF3D7_1477700 and PF3D7_1438800 transcript-positive gametocytes as detected by stage-specific reverse transcriptase quantitative PCR: More than half of the participants were positive for PF3D7_1477700 mRNA, but not all these were positive for PF3D7_1438800 mRNA levels suggesting the presence of early maturing gametocytes. An even smaller proportion was positive for both PF3D7_1477700 and PF3D7_1438800 transcripts. This figure appears in color at www.ajtmh.org.
Table 2.
Clinical, demographic parameters and the composition of gametocytes as measured by reverse transcriptase quantitative PCR
| Parameter | Early-mid gametocytes (N = 38) | % Early-mid gametocytes | Mid-late gametocytes (N = 26) | % Mid-late gametocytes | |
|---|---|---|---|---|---|
| Gender | M | 18 | 47 | 13 | 50 |
| F | 20 | 53 | 13 | 50 | |
| Fever | Yes | 15 | 39 | 7 | 27 |
| No | 23 | 63 | 19 | 73 | |
| Age | < 5 years | 6 | 16 | 6 | 23 |
| 5–10 years | 12 | 32 | 8 | 31 | |
| 10–16 years | 9 | 24 | 7 | 27 | |
| > 16 years | 11 | 29 | 5 | 19 | |
| Anemia | Moderate | 16 | 42 | 11 | 42 |
| Mild | 16 | 42 | 11 | 42 | |
| Non-anemic | 6 | 16 | 4 | 15 |
M represents males whereas F represents females.
Prevalence of antimalarial (SNPs).
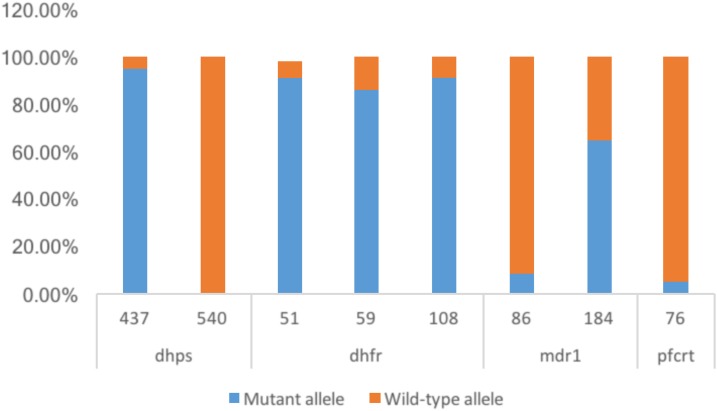
The prevalence of known drug resistance–associated gene mutations in the parasite isolates was determined and related to gametocyte carriage in the participants. No isolate was found to contain the dhps-540 mutant allele, but the IRN triple mutant of pfdhfr was the predominant genotype at this locus (Figure 2).
Figure 2.
Prevalence of antimalarial drug resistance-associated single nucleotide polymorphisms (SNPs): Each bar represents the proportion of the samples that display the mutant (blue) or wild-type (orange) amino acid at the position designated below the bar. Besides Plasmodium falciparum chloroquine resistance transporter (pfcrt)-76 and P. falciparum multidrug resistance gene one (Pfmdr1)-86 with mutations less than 10%, all other SNPs that we investigated were found to be saturated. No mutation in dhps-540 was found. This figure appears in color at www.ajtmh.org.
Sixty percent (3/5) and 48% (19/31) of the participants that harbored mutations in mdr1-86 and mdr1-184, respectively, were positive for DG as determined by RT-qPCR. Similar observations were made when the prevalence of mdr1-86 and mdr1-184 mutations were compared with individuals harboring mature gametocytes (Table 3). Fifty-seven percent of the participants with the mutation dhps-437 had developing and mature gametocytes. All three mutations of dhfr tested were quite high, dhfr-51, dhfr-59, and dhfr-108, 91.07% (51/56), 85.96% (49/57), and 91.07% (51/56), respectively (Figure 2). Because of the high prevalence of the respective dhfr mutations, between 40% and 60% of participants expressing the different gametocyte markers had the mutations as seen already for dhps (Table 3).
Table 3.
Antimalaria-associated SNPs and the compositions of gametocytes
| SNP | Number mutant | Early-mid gametocytes (N = 38) | % Early-mid gametocytes | Mid-late gametocytes (N = 26) | % Mid-late gametocytes |
|---|---|---|---|---|---|
| dhps_gly437 | 56 | 32 | 84 | 22 | 85 |
| dhfr_asn51ile | 51 | 28 | 74 | 20 | 77 |
| dhfr_cys59arg | 49 | 29 | 76 | 21 | 81 |
| dhfr_asn108 | 51 | 28 | 74 | 20 | 77 |
| mdr1_n86y | 5 | 3 | 8 | 3 | 12 |
| mdr1_y184f | 39 | 19 | 50 | 13 | 50 |
| pfcrt_k76t | 3 | 2 | 5 | 0 | 0 |
pfcrt = Plasmodium falciparum chloroquine resistance transporter; SNP = single nucleotide polymorphism.
Age, hemoglobin levels, fever, and gametocytemia.
Anemia was very common among our study participants. Based on the World Health Organization definition of anemia,35 we found that 87.5% of all our study participants had various degrees of anemia ranging from severe to mild anemia. Based on a linear logistic regression model, we observed that study participants between the ages of five (odds ratio [OR] = 4.50, 95% CI: 0.99–21.11) and 16 (OR = 5.00, 95% CI: 1.02–24.27) (Table 4) were more likely to carry MG transcripts compared with those younger than 5 years or older than 16 years. Moreover, mild anemia (OR = 1.88 [95% CI: 0.69–5.11] and no anemia (OR = 2.77 [95% CI: 0.56–13.76] (Table 4) were possible predictors of gametocytemia compared with severely anemic participants. The prevalence of gametocytemia was less likely in patients with fever (OR = 0.74 [95% CI: 0.28–1.97] as compared with those without fever (Table 4). When the early-mid gametocyte marker was used, only age was found to be associated with gametocytemia (5–9 years: OR = 3.27 CI: 0.65–16.38 and 10–16 years: OR = 2.05 CI: 0.44–9.58).
Table 4.
Possible predictors of gametocyte carriage among study participants: Using linear logistic regression, individuals between the ages of 5 and 16 years were more likely to carry gametocytes
| Risk factors for gametocytemia | Odds ratio (95% CI) | |
|---|---|---|
| Age | < 5 years | – |
| 5–10 years | 4.50 (0.99–21.11) | |
| 10–16 years | 5.00 (1.02–24.27) | |
| > 16 years | 1.32 (0.32–5.43) | |
| Fever | No | – |
| Yes | 0.74 (0.28–1.97) | |
| Hemoglobin | Moderate anemia | – |
| Mild anemia | 1.88 (0.69–5.11) | |
| Non-anemic | 2.77 (0.56–13.76) |
Gametocytes were more prevalent in individuals without a history of fever and participants with mild anemia or those non-anemic had a higher chance of gametocytemia as compared with severe or moderately anemic participants. Severely anemic participants were included in the moderate anemic participants for analysis because they formed a small group. Anemia was defined as severe (< 8 g/dL), moderate (8–11 g/dL), mild (11–13 g/dL) and non-anemic (> 13 g/dL).
DISCUSSION
The present study identified age, fever, and hemoglobin levels to be associated with carriage of MG transcripts, which may be a useful indicator of gametocyte carriage in malaria patients. Alleles associated with resistance to various antimalarial drugs were very common, including among participants with RT-qPCR evidence of gametocyte carriage. We also found higher estimates of gametocyte carriage by RT-qPCR than microscopy and showed that some individuals with DG mRNA did not have detectable MG mRNA levels. This was surprising because DG mRNA is assumed to indicate the presence of immature gametocytes, which are sequestered in tissues such as the bone marrow and therefore should not be detected in samples from the peripheral circulation of patients. However, in vitro studies have shown that DG’ transcripts can possibly come from sexually committed TR as they begin the asexual–sexual stage transition.22 Therefore, these transcripts could indeed represent asexual parasites from the gametocyte commitment cycle which occurs before sexual-stage induction and differentiation. A longitudinal study is required to investigate the possibility of sexually committed TR in the peripheral circulation and their potential disappearance through sequestration.
This study was able to identify 36% of participants as carriers of mature gametocytes through the detection of MG transcripts. In comparison, only 12.5% of individuals were identified as gametocyte carriers by microscopy. Several studies using molecular tools similarly detected higher levels of submicroscopic densities of gametocytes when compared with microscopy, and the gametocytes detected were even shown to establish infection in mosquitoes.36–38 In most of these studies, higher gametocyte densities were observed after drug treatment compared with the time of clinical presentation. artemisinin-based therapies reduce submicroscopic gametocytemia but do not completely prevent posttreatment transmission to mosquitoes in other studies.39 Our study participants were not followed up after treatment and it was not possible to estimate the level of submicroscopic gametocytemia following treatment. However, it is evident that microscopy heavily underestimates the proportion of individuals potentially capable of transmitting gametocytes to mosquitoes.
Many factors are considered to influence commitment and production of gametocytes including genetic switching22,23 and environmental factors.40–42 Supplementation of serum, regardless of the malaria infection status of donors, has been shown to induce and improve gametocyte yields in in vitro gametocyte cultures.9,43 Clinical parameters such as age, the absence of fever, and hemoglobin levels have been previously shown to influence the degree of gametocytemia in individuals treated with different types of antimalarials.11,44–46 We found no strong relationship between age, hemoglobin levels, or fever with gametocytemia, but given our small sample size, these relationships will be investigated in larger studies in the future. However, the association we see with higher gametocyte prevalence in slightly older participants could be due to the level of clinical immunity they have acquired. Clinical immunity could play several roles including maintenance of low-grade asexual parasitemia and constant modulation of sexual development. Hemolysis of erythrocytes because of either high burden of parasitemia or effects of drugs have been suggested to trigger gametocyte production, but the degree of hemolysis was not investigated.47 This is consistent with our observation of gametocytemia in mildly anemic participants and agrees with other findings on the clinical correlates of gametocytemia.46 The high odds ratios for non-anemic, fever-free individuals is interesting and may suggest high prevalence of gametocytes in asymptomatics as already shown in other studies.46
Additional factors associated with patient gametocyte carriage include slow parasite clearance and carriage of drug resistance genotypes/alleles/polymorphisms.48,49 We found very high antimalarial-associated SNPs in some isolates but not others. Higher levels of dhps and dhfr alleles, which are associated with sulfadoxine–pyrimethamine (SP) resistance, were observed, but parasites carrying the crucial pfdhps-540E allele were absent among our study participants. In Ghana, SP is used for intermittent preventive treatment of malaria in pregnant women, which could contribute to the moderate levels of predicted SP resistance we and others have observed.50 On the contrary, low levels of chloroquine resistance–associated alleles were detected and may be an indication of the parasite population reverting to the sensitive genotype on removal of chloroquine from the market, as has been seen in other places.51 We however, did not find any significant correlation of the presence of these SNPs and gametocyte-specific transcripts in our patients probably because of the fact that most of the SNPs had a very high prevalence, and so our study was underpowered to test for such associations.
CONCLUSION
This study provides further evidence of the relationship between hemoglobin levels and the age of malaria patients and gametocyte carriage. In addition, it demonstrates an abundance of submicroscopic gametocytes as measured by RT-qPCR in untreated but sick populations providing ample opportunity for malaria transmission before treatment. The possibility that DG transcripts are present in sexually committed TR should be investigated in future studies.
Supplementary Material
Acknowledgments:
We are most grateful to the participants and guardians for making the study possible. We are also very grateful to Mathias Marti of the University of Glasgow, United Kingdom, for helpful discussions on reverse transcriptase quantitative PCR. Prince Nyarko of the West African Centre for Cell Biology of Infectious Pathogens is also thanked for help with handling clinical isolates.
Note: Supplemental text appears at www.ajtmh.org.
REFERENCES
- 1.WHO , 2017. World Malaria Report 2017 Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/malaria/media/world-malaria-report-2017/en/. Accessed February 19, 2018.
- 2.Dinko B, Ayivor-Djanie R, Abugri J, Agboli E, Kye-Duodu G, Tagboto S, Tampuori J, Adzaku F, Binka FN, Awandare GA, 2016. Comparison of malaria diagnostic methods in four hospitals in the Volta region of Ghana. MalariaWorld J 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smalley ME, Abdalla S, Brown J, 1981. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg 75: 103–105. [DOI] [PubMed] [Google Scholar]
- 4.Carter R, Graves PM, 1988. Gametocytes. Wernsdorfer WH, McGregor I, eds. Malaria: Principles and Practice of Malariology. London, United Kingdom: Churchill Livingstone, 253–320. [Google Scholar]
- 5.Alano P, 2007. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol 66: 291–302. [DOI] [PubMed] [Google Scholar]
- 6.Joice R, et al. 2014. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med 6: 244re5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawking F, Wilson ME, Gammage K, 1971. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg 65: 549–559. [DOI] [PubMed] [Google Scholar]
- 8.Sinden RE, Carter R, Drakeley C, Leroy D, 2012. The biology of sexual development of Plasmodium: the design and implementation of transmission-blocking strategies. Malar J 11: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebru T, Lalremruata A, Kremsner PG, Mordmüller B, Held J, 2017. Life-span of in vitro differentiated Plasmodium falciparum gametocytes. Malar J 16: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K, 2001. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans R Soc Trop Med Hyg 95: 497–501. [DOI] [PubMed] [Google Scholar]
- 11.Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, ter Kuile F, van Vugt M, Chongsuphajaisiddhi T, White NJ, 1999. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg 60: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 12.Gouagna LC, Bancone G, Yao F, Yameogo B, Dabiré KR, Costantini C, Simporé J, Ouedraogo JB, Modiano D, 2010. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat Genet 42: 328–331. [DOI] [PubMed] [Google Scholar]
- 13.Bousema T, Drakeley C, 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24: 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drakeley CJ, Secka I, Correa S, Greenwood BM, Targett GA, 1999. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Trop Med Int Health 4: 131–138. [DOI] [PubMed] [Google Scholar]
- 15.Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, Roper C, Watkins B, White NJ, 2008. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J Infect Dis 197: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 16.Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GA, 2006. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol 22: 424–430. [DOI] [PubMed] [Google Scholar]
- 17.Lobo CA, Kumar K, 1998. Sexual differentiation and development in the malaria parasite. Parasitol Today 14: 146–150. [DOI] [PubMed] [Google Scholar]
- 18.Nantakomol D, et al. 2011. Circulating red cell-derived microparticles in human malaria. J Infect Dis 203: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel PY, et al. 2013. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regev-Rudzki N, et al. 2013. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153: 1120–1133. [DOI] [PubMed] [Google Scholar]
- 21.Brancucci NMB, et al. 2017. Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell 171: 1532–1544.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC, 2005. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol 143: 90–99. [DOI] [PubMed] [Google Scholar]
- 23.Eksi S, et al. 2012. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog 8: e1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kafsack BF, et al. 2014. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507: 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha A, et al. 2014. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 507: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joice R, et al. 2013. Inferring developmental stage composition from gene expression in human malaria. PLoS Comput Biol 9: e1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plasmo DB, 2018. The Plasmodium Genome Resources Available at: http://plasmodb.org/plasmo/. Accessed January 12, 2018.
- 28.Kweku M, Liu D, Adjuik M, Binka F, Seidu M, Greenwood B, Chandramohan D, 2008. Seasonal intermittent preventive treatment for the prevention of anaemia and malaria in Ghanaian children: a randomized, placebo controlled trial. PLoS One 3: e4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwood BM, Armstrong JR, 1991. Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg 85: 186–188. [DOI] [PubMed] [Google Scholar]
- 30.Drakeley CJ, Eling W, Teelen K, Bousema JT, Sauerwein R, Greenwood BM, Targett GA, 2004. Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol 26: 159–165. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, 1993. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15: 532–537. [PubMed] [Google Scholar]
- 32.Djimdé A, et al. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344: 257–263. [DOI] [PubMed] [Google Scholar]
- 33.Duraisingh MT, Curtis J, Warhurst DC, 1998. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp Parasitol 89: 1–8. [DOI] [PubMed] [Google Scholar]
- 34.Chang HH, et al. 2016. Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci Rep 6: 26330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO , 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity, Report, 2011 Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed August 14, 2017.
- 36.Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, Sauerwein RW, 2007. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg 76: 470–474. [PubMed] [Google Scholar]
- 37.Bousema JT, et al. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis 193: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 38.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, Basáñez MG, 2013. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife 2: e00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targett G, et al. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis 183: 1254–1259. [DOI] [PubMed] [Google Scholar]
- 40.Carter R, Miller LH, 1979. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ 57 (Suppl 1): 37–52. [PMC free article] [PubMed] [Google Scholar]
- 41.Graves PM, Carter R, McNeill KM, 1984. Gametocyte production in cloned lines of Plasmodium falciparum. Am J Trop Med Hyg 33: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 42.Dyer M, Day KP, 2000. Commitment to gametocytogenesis in Plasmodium falciparum. Parasitol Today 16: 102–107. [DOI] [PubMed] [Google Scholar]
- 43.Smalley ME, Brown J, 1981. Plasmodium falciparum gametocytogenesis stimulated by lymphocytes and serum from infected Gambian children. Trans R Soc Trop Med Hyg 75: 316–317. [DOI] [PubMed] [Google Scholar]
- 44.Meerman L, Ord R, Bousema JT, van Niekerk M, Osman E, Hallett R, Pinder M, Walraven G, Sutherland CJ, 2005. Carriage of chloroquine-resistant parasites and delay of effective treatment increase the risk of severe malaria in Gambian children. J Infect Dis 192: 1651–1657. [DOI] [PubMed] [Google Scholar]
- 45.Dunyo S, Milligan P, Edwards T, Sutherland C, Targett G, Pinder M, 2006. Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PLoS Clin Trials 1: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WWARN Gametocyte Study Group , 2016. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med 14: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieckmann KH, McNamara JV, Kass L, Powell RD, 1969. Gametocytocidal and sporontocidal effects of primaquine upon two strains of Plasmodium falciparum. Mil Med 134: 802–819. [PubMed] [Google Scholar]
- 48.Ashley EA, et al. Tracking Resistance to Artemisinin Collaboration (TRAC) , 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beshir KB, et al. 2013. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis 208: 2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venkatesan M, Alifrangis M, Roper C, Plowe CV, 2013. Monitoring antifolate resistance in intermittent preventive therapy for malaria. Trends Parasitol 29: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiarie WC, Wangai L, Agola E, Kimani FT, Hungu C, 2015. Chloroquine sensitivity: diminished prevalence of chloroquine-resistant gene marker pfcrt-76 13 years after cessation of chloroquine use in Msambweni, Kenya. Malar J 14: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.