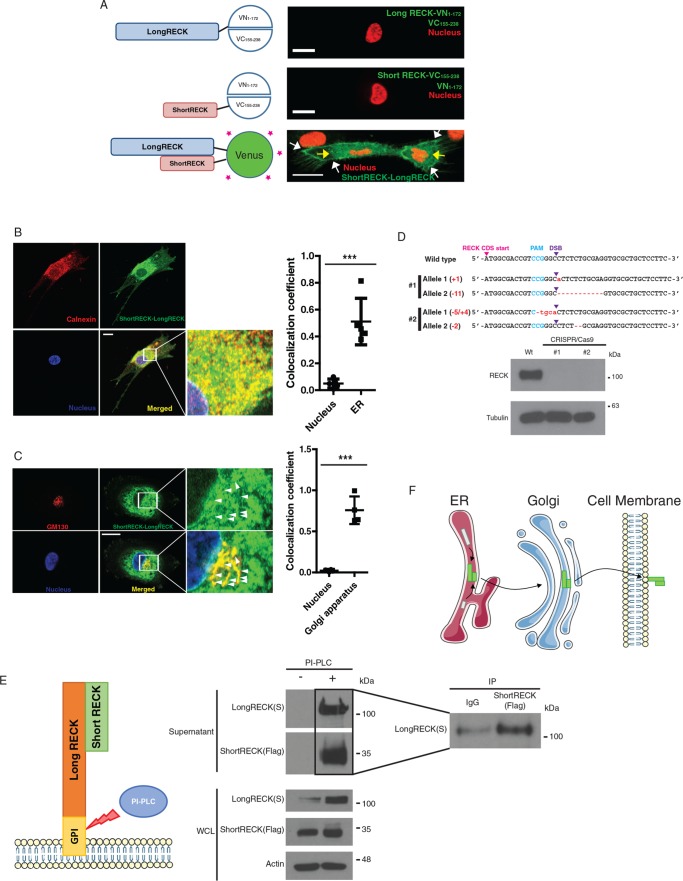
FIGURE 4:
A short RECK–long RECK complex is localized in the perinuclear region and on the cell surface. (A) The N-terminal half of Venus (1–172) was conjugated to the long RECK isoform, and the C-terminal half of Venus (155–238) was conjugated to the short RECK isoform for bimolecular fluorescence complementary (BiFC) assays. When either long RECK-VN and free VC or short RECK-VC and free VN were overexpressed, no green signal was observed (top and middle). When both long RECK-VN and short RECK-VC were overexpressed in the same cell, a green signal was observed on the cell surface (white arrows) and in the perinuclear region (yellow arrows; bottom). The scale bar indicates 20 μm. (B) Immunofluorescence staining of short RECK-long RECK BiFC cells (green) with calnexin, an ER marker (red). About 50% of the short RECK–long RECK interaction signal overlaps with calnexin. The scale bar indicates 20 μm. Averages and SD are shown. p < 0.001, unpaired two-sided Student’s t test. (C) Immunofluorescence staining of short RECK–long RECK BiFC cells with GM130, a Golgi apparatus marker. The green signal indicating tubule structures around the nucleus (white arrowheads) overlapped with the red GM130 signal to produce a merged yellow signal. About 70% of GM130 overlaps with the short RECK–long RECK interaction signal. The scale bar indicates 20 μm. Averages and SD are shown. p < 0.001, unpaired two-sided Student’s t test. (D) Characterization of a RECK knockout HEK293T cell line established by CRISPR/Cas9. Top panel, sequencing results from two different RECK knockout cell lines. Frameshift mutations were introduced into the RECK sequence by introducing Cas9 endonuclease and a guide RNA targeting RECK (PAM, protospacer adjacent motif; DSB, double-strand break). Bottom panel, Western blot results showing knockout of RECK in CRISPR/Cas9-engineered 293Ts. (E) Left panel, strategy to detect short RECK–long RECK complex on the cell surface. PI-PLC cleaves the GPI anchor attaching long RECK to the cell surface, releasing the complex into the supernatant. Middle panel, Western blot results showing the release of short RECK on the cell surface by PI-PLC treatment. The short RECK isoform, which does not have a GPI anchor, is liberated from the cell surface when long RECK is cleaved from the cell surface by PI-PLC. Right panel, liberated short RECK and long RECK isoforms in the supernatant interact based on coimmunoprecipitation. WCL, whole cell lysate. (F) Hypothesized model for the trafficking of a short RECK–long RECK complex. According to the model, short RECK–long RECK complexes are formed in the ER and then transported to the cell surface via the Golgi apparatus.