Abstract.
The World Health Organization has warned that substandard and falsified medical products (SFs) can harm patients and fail to treat the diseases for which they were intended, and they affect every region of the world, leading to loss of confidence in medicines, health-care providers, and health systems. Therefore, development of analytical procedures to detect SFs is extremely important. In this study, we investigated the quality of pharmaceutical tablets containing the antihypertensive candesartan cilexetil, collected in China, Indonesia, Japan, and Myanmar, using the Japanese pharmacopeial analytical procedures for quality control, together with principal component analysis (PCA) of Raman spectrum obtained with handheld Raman spectrometer. Some samples showed delayed dissolution and failed to meet the pharmacopeial specification, whereas others failed the assay test. These products appeared to be substandard. Principal component analysis showed that all Raman spectra could be explained in terms of two components: the amount of the active pharmaceutical ingredient and the kinds of excipients. Principal component analysis score plot indicated one substandard, and the falsified tablets have similar principal components in Raman spectra, in contrast to authentic products. The locations of samples within the PCA score plot varied according to the source country, suggesting that manufacturers in different countries use different excipients. Our results indicate that the handheld Raman device will be useful for detection of SFs in the field. Principal component analysis of that Raman data clarify the difference in chemical properties between good quality products and SFs that circulate in the Asian market.
INTRODUCTION
In May 2017, definition of substandard and falsified medical products (SFs) was announced by the World Health Organization.1 Substandard medical products (also called “out of specification”) are authorized by national regulatory authorities but fail to meet either national or international quality standards or specifications, or in some cases, both. On the other hand, falsified medical products deliberately or fraudulently misrepresent their identity, composition, or source.1–5 Many surveys of falsified medical products and analytical procedures for investigation of the authenticity of medical products have been reported by various public institutes.2–12 In 2015, the Pharmaceutical Security Research Institute reported that Asia experienced the highest incidence of drug crime cases among seven regions in the world. In that year, a total of 3,002 cases of drug crime cases were recorded, among which around 1,000 involved the Asia-Pacific region.13 Many cases where defective products have been transported across national borders have been reported.14
Relatively little work has been carried out on analytical methods for investigating the actual status of substandard medical products, including their distribution, and their physical and chemical properties.14,15 One reason for this maybe concern about the possibility of excessively hindering the development of medicines and access to medicines in developing countries.16 Also, regular quality control and surveillance of medicines after marketing tend to be more difficult in developing countries for various reasons, including high cost, the need for sophisticated equipment and skilled technicians, and lack of pharmacological knowledge to recognize the need for implementation of countermeasures.6,15,17–19 Furthermore, medicines may be transported across national borders without proper quality checks through various distribution channels.14,20 These are serious issues to be taken measure of, because SFs can cause treatment failure, development of antimicrobial resistance, and serious adverse drug reactions, thereby damaging public confidence in medicines.2,21,22
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q6A provides guidance to establish a harmonized set of global specifications consisting of analytical procedures and acceptance criteria for new drug substances (DS) and drug products (DP) for human use (1999).23 Specifications of DS and DP are proposed and justified by the manufacturer, and approved by regulatory authorities in each country. The specifications and acceptance criteria are focused on those chemical, physical, and biological properties considered to be important for ensuring the safety and efficacy of DS and DP. Thus, they can be adopted to identify substandard products. Possible issues include 1) out-of-specification content of active pharmaceutical ingredient (API),24,25 2) significant dissolution delay,24 3) contamination with toxic substances,26,27 and 4) lack of sterility.28,29 These points can be checked by means of assay, content uniformity testing, measurements of dissolution properties and impurities, and microbial tests.
In this study, candesartan cilexetil tablets were collected in China, Indonesia, Japan, and Myanmar and subjected to quality control tests (assays, content uniformity, and dissolution tests) according to the Japanese pharmacopeia.30 The acceptance criteria for these tests in the Japanese pharmacopeia were adopted as thresholds for identification of SFs.
Many issues of quality and bioavailability are considered to be due to technical deficiencies in the manufacturing process design and differences in the nature of the excipients.31–33 Although many studies have shown that excipients influence quality, the excipients are not generally stipulated in quality tests. Our previous study found that the types of the excipients used in candesartan cilexetil tablets differ depending on the manufacturer, and Raman spectra of the tablets showed the different pattern reflecting the chemical nature of the excipients.34 Here, we focused on the methodology for detecting substandard and falsified medicines by principal component analysis (PCA)12,22,35–43 of raw data obtained by handheld Raman spectroscopy. We aimed to clarify the chemical features of substandard medicines by comparing them with authentic medicines, and by extracting the principal components of the Raman spectrum to visualize the relationships among the tablets. We chose the handheld Raman device as a simple spectroscope suitable for in situ observation in developing countries, and we used PCA as a mean to extract critical information despite the limited resolution and sensitivity of the device. We also compared signal preprocessing methods of Raman spectra for PCA and selected the multiplicative scattering correlation (MSC) method as being particularly suitable to extract the desired signals from the strong fluorescence background.43 This approach proved highly effective to evaluate the degree of similarity among samples.
EXPERIMENTAL
Sample collection.
Authentic candesartan cilexetil tablets (Blopress 4 mg, 8 mg and 12 mg) and placebo tablets including the excipients same as Blopress except API were supplied by a Japanese manufacturer (Takeda Pharmaceutical Company Ltd., Osaka, Japan) as the reference samples. Samples of candesartan cilexetil tablets were collected from the hospitals and clinics in China, Indonesia, and Myanmar and also purchased via the internet (2009–2015). The falsified products imitating Blopress discovered in Indonesia have been reported to Forensics, Brand Protection, and Investigations. These falsified products were identified based on visual inspection of the packaging.34
Visual inspection.
First, we observed the outer package and package insert, Press Through Pack, or aluminum blister packaging. The product name, dose, component, formulation, packaging unit, manufacturer, manufacturing date, expiration date, and manufacturing number were recorded. The cartons were examined visually and microscopically and compared with reference samples. Printing on the edge of the tape seal was carefully observed to check fine details.
Quality control test.
Content uniformity,44 assay, and dissolution tests of candesartan cilexetil tablets were conducted according to Japanese pharmacopeia.30
Acetonitrile (for high-performance liquid chromatography [HPLC]), polyoxyethylene 20, and sorbitan monolaurate (for biochemistry) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), acetic acid, and acenaphthene were purchased from Nacalai Tesque Co., Ltd (Kyoto, Japan).
Content uniformity and assay.
Candesartan cilexetil in tablets were extracted in a mixture of acetonitrile and water (3:2) and measurements were carried out at a wavelength of 305 nm using a spectrophotometer (U-3210, HITACHI, Tokyo, Japan). Because the number of the collected samples was limited, two, three, or 10 tablets were used for each evaluation of content uniformity and the mean of the content was calculated as the result for assay. The acceptance criterion for the assay was set to 95.0–105.0% as same as the criterion in Japanese Pharmacopeia.30
Dissolution.
The dissolution test was performed under the condition described for Apparatus 2 (paddle method) with 50-rpm agitation in 900 mL of a dissolution medium containing 10 w/v% polyoxyethylene (20) sorbitan monolaurate at 37°C. A sample was taken at the time point of 45 minutes, and examined by high-speed liquid chromatography (HPLC, L-7200 autosampler, D-7000 interface, L-7100 pump, L-7300 column, L-7405 UV detector, HITACHI, Tokyo, Japan). Acenaphthene was added to the test solution as the internal standard. High-performance liquid chromatography conditions were as follows: 5-μm-octa decyl silyl (ODS) column (Shim-pack CLC-ODS (M) 15 cm, SHIMADZU, Kyoto, Japan), a flow rate of 1.8 mL/minute, a column temperature of 25°C, an injection volume of 50 μL, a detection wavelength of 254 nm. The mobile phase was a mixture of acetonitrile, water, and acetic acid (57:43:1). The mean of the dissolution rate of two, three, or six tablets were evaluated, respectively. The criterion was set that the dissolution rate of candesartan cilexetil should be more than 75% at the 45-minute sampling point.30
Handheld Raman spectroscopy.
All tablets were examined with a handheld Raman spectrometer (TruScan®, Thermo Fisher Scientific, Waltham, MA). The chemical equivalence between the authentic reference product (tablets containing 8 mg API) and the collected samples was evaluated based on the P value for similarity of the Raman spectra, which was automatically calculated by the instrument’s built-in algorithm, which has been validated, but not disclosed, and is designed not to be modified. Next, the raw Raman data were subjected to PCA analysis. Spectra components from the API were obtained by analysis of authentic tablets containing 0 mg (Placebo), 4 mg, 8 mg, and 12 mg of the API. The weight and size of 0 mg (Placebo), 4 mg, 8 mg, and 12 mg tablets are equivalent, and same weights of each excipient except lactose monohydrate to adjust total weights of them.
Preprocessing of Raman spectra.
The data interval of the handheld device is around 1.4–2.2 cm−1 and the noise level is high, so preprocessing of the spectroscopy spectra is critical for accurate PCA calculation. We used the Savitzky–Golay (SG) method45 to smooth each segment of the original Raman spectrum in a small window by fitting to a polynomial function.45,46 The MSC method46–49 was also applied to eliminate the baseline shift caused by the multiplication of the baseline tilts and the additive shift of the baseline shifts up and down.46–49 Multiplicative scattering correlation can use data from many wavelengths to distinguish between light absorption and light scattering, correcting spectra according to a simple linear univariate fit to a standard spectrum by means of least-squares regression using the standard spectrum.47 The observed spectrum is considered to depend on wavelength as follows:
| (1.1) |
where, is the standard spectrum and represents the residual. a and b are adjusted to minimize the term of e(ω), to make these discrete deviations as small as possible.47
Principal component analysis.
The Unscrambler® X software (CAMO Software, Oslo, Norway) was used for PCA. The Raman spectra data set consisting of 85 samples and 476 wavenumbers was calculated and it was decomposed into a linear combination of score tn and loading pn consisting of several principal components, allowing the spectrum to be understood clearly with a limited number of principal components. That is, the data set X is decomposed into a linear combination of the score and the loading as shown in equation (1.2).
| (1.2) |
The validity and robustness of the calculated PCA model were confirmed by cross-random validation.
RESULTS
Table 1 shows the summary of collected samples from China, Indonesia, and Myanmar. More than 15 brands of candesartan cilexetil tablets are available in China, as judged from an internet survey, but only four were found to be distributed in hospitals and clinics in Shanghai. The products distributed in Shanghai in China were from manufacturers in China and Japan. On the other hand, three brands, manufactured in Japan, India, and Pakistan, were found in private hospitals, community pharmacies, and wholesalers in Mandalay, Myanmar. No obvious deficiencies in the PTP packaging, package insert for use, pillows, or tablets were found in visual inspection of all collected samples.
Table 1.
Summary of collected samples from China, Indonesia, and Myanmar
| No. | Product name stated on the package | Dose (mg) | Manufacturing country printed on package | Sampling area | Sampling year | Sampling facility |
|---|---|---|---|---|---|---|
| 1–7 | Blopress | 8 | Japan | Shanghai, China | 2012 | Hospital 1–7 |
| 8–9 | 悉君宁 | 4 | China | Shanghai, China | 2012 | Hospital 8–9 |
| 10–11, 13 | 维尔亚 | 8 | China | Shanghai, China | 2012 | Hospital 10–11, 13 |
| 12 | 维尔亚 | 4 | China | Shanghai, China | 2012 | Hospital 12 |
| 14–26 | XINXIN | 4 | China | Shanghai, China | 2012 | Hospital 14–26 |
| 27–30 | Candelong-8 | 8 | India | Mandalay, Myanmar | 2015 | Pharmacy A–D |
| 31–33 | Advant | 8 | Pakistan | Mandalay, Myanmar | 2015 | Pharmacy E–G |
| 34 | Falsified product imitating Blopress | 16 | Indonesia | Medan, Indonesia | 2009 | Pharmacy H |
| 35, 37 | Falsified product imitating Blopress | 16 | Indonesia | Jakarta, Indonesia | 2011–2012 | Pharmacy I, J |
| 36 | Falsified product imitating Blopress | 8 | Indonesia | Jakarta, Indonesia | 2011 | Pharmacy I |
Content uniformity, assay and dissolution.
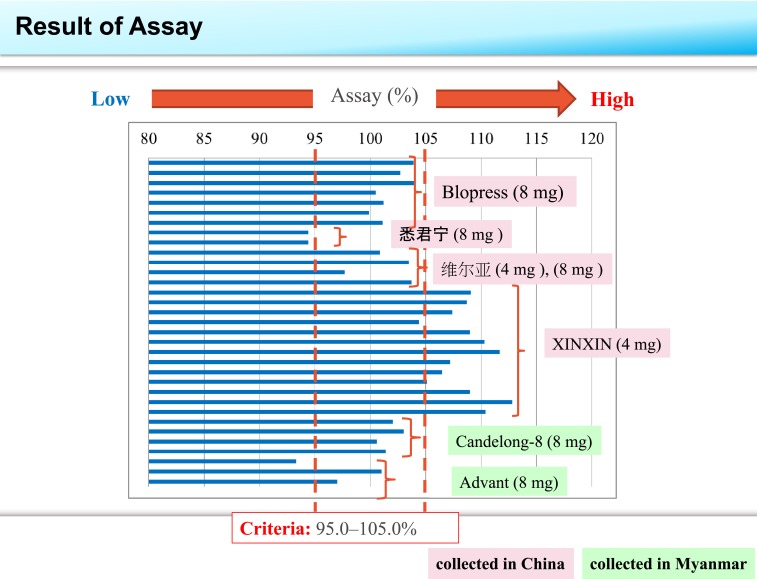
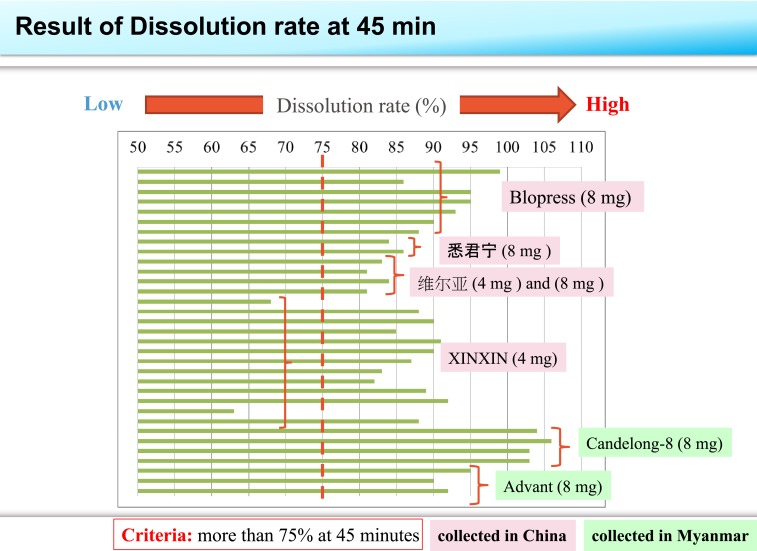
Table 2 summarizes the results of the content uniformity, assay, and dissolution tests of collected samples. The assay values of 12 of 13 samples of the tablets stated as XINXIN candesartan cilexetil tablets exceeded the upper limit of 105.0%. Two samples stated as 悉 君 宁 and one sample stated as Advant candesartan cilexetil tablets gave an assay value less than the lower acceptance limit of 95.0% as shown in Figure 1. Dissolution delay was confirmed in two samples of XINXIN candesartan cilexetil tablets, which failed to meet the criterion dissolution rate of more than 75% at 45 minutes as shown in Figure 2. Other tablets met the criterion.
Table 2.
Summary results of the assay, content uniformity and dissolution tests of candesartan cilexetil tablets, and chemical similarity based on Raman spectra
| No. | Stated product name | Dose (mg) | Dissolution | Assay | Chemical similarity by Raman spectra | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean of dissolution rate (%) | Standard deviation | Number of tablets | Judgment | Mean of content (%) | Standard deviation | Number of tablets | Judgment | ||||
| 1 | Blopress | 8 | 99 | 1.9 | 3 | Pass | 103.9 | 0.4 | 3 | Pass | 0.7159 |
| 2 | Blopress | 8 | 86 | 0.6 | 3 | Pass | 102.7 | 3.3 | 3 | Pass | 0.5689 |
| 3 | Blopress | 8 | 95 | 0.4 | 3 | Pass | 104.0 | 0.4 | 3 | Pass | 0.6546 |
| 4 | Blopress | 8 | 95 | 0.4 | 3 | Pass | 100.5 | 0.1 | 3 | Pass | 0.6914 |
| 5 | Blopress | 8 | 93 | 2.2 | 2 | Pass | 101.2 | 0.6 | 3 | Pass | 0.6763 |
| 6 | Blopress | 8 | 90 | 1.8 | 3 | Pass | 99.9 | 0.7 | 3 | Pass | 0.5910 |
| 7 | Blopress | 8 | 88 | 1.3 | 3 | Pass | 101.1 | 1.7 | 3 | Pass | 0.6158 |
| 8 | 悉君宁 | 4 | 84 | 0.6 | 3 | Pass | 94.4 | 2.2 | 3 | Fail | 0.0000 |
| 9 | 悉君宁 | 4 | 86 | 3.1 | 3 | Pass | 94.4 | 2.1 | 3 | Fail | 0.0000 |
| 10 | 维尔亚 | 8 | 83 | 1.5 | 3 | Pass | 100.9 | 1.5 | 3 | Pass | 0.0000 |
| 11 | 维尔亚 | 8 | 81 | 4.1 | 3 | Pass | 103.5 | 4.8 | 3 | Pass | 0.0000 |
| 12 | 维尔亚 | 4 | 84 | 0.2 | 3 | Pass | 97.7 | 0.6 | 3 | Pass | 0.1483 |
| 13 | 维尔亚 | 8 | 81 | 4.0 | 3 | Pass | 103.7 | 0.8 | 3 | Pass | 0.0000 |
| 14 | XINXIN | 4 | 68 | 15.9 | 3 | Fail | 109.1 | 1.6 | 3 | Fail | 0.0000 |
| 15 | XINXIN | 4 | 88 | 3.8 | 3 | Pass | 108.7 | 1.8 | 3 | Fail | 0.0000 |
| 16 | XINXIN | 4 | 90 | 4.1 | 3 | Pass | 107.4 | 2.1 | 3 | Fail | 0.0000 |
| 17 | XINXIN | 4 | 85 | 1.0 | 3 | Pass | 104.4 | 2.5 | 3 | Pass | 0.0000 |
| 18 | XINXIN | 4 | 91 | 0.2 | 3 | Pass | 109.0 | 2.7 | 3 | Fail | 0.0000 |
| 19 | XINXIN | 4 | 90 | 4.4 | 3 | Pass | 110.3 | 0.4 | 3 | Fail | 0.0000 |
| 20 | XINXIN | 4 | 87 | 4.4 | 3 | Pass | 111.7 | 1.2 | 3 | Fail | 0.0000 |
| 21 | XINXIN | 4 | 83 | 1.2 | 3 | Pass | 107.2 | 4.8 | 3 | Fail | 0.0000 |
| 22 | XINXIN | 4 | 82 | 1.2 | 3 | Pass | 106.5 | 2.5 | 3 | Fail | 0.0000 |
| 23 | XINXIN | 4 | 89 | 3.1 | 3 | Pass | 105.1 | 1.8 | 3 | Fail | 0.0000 |
| 24 | XINXIN | 4 | 92 | 0.1 | 3 | Pass | 109.0 | 1.1 | 3 | Fail | 0.0000 |
| 25 | XINXIN | 4 | 63 | 13.3 | 3 | Fail | 112.8 | 4.0 | 3 | Fail | 0.0000 |
| 26 | XINXIN | 4 | 88 | 0.9 | 3 | Pass | 110.4 | 1.8 | 3 | Fail | 0.0000 |
| 27 | Candelong-8 | 8 | 104 | 0.4 | 6 | Pass | 102.0 | 1.2 | 10 | Pass | 0.0337 |
| 28 | Candelong-8 | 8 | 106 | 0.2 | 3 | Pass | 103.0 | 0.3 | 3 | Pass | 0.0969 |
| 29 | Candelong-8 | 8 | 103 | 2.9 | 6 | Pass | 100.6 | 2.2 | 10 | Pass | 0.0457 |
| 30 | Candelong-8 | 8 | 103 | 1.1 | 6 | Pass | 101.4 | 2.1 | 10 | Pass | 0.0302 |
| 31 | Advant | 8 | 95 | 2.9 | 6 | Pass | 93.3 | 3.8 | 10 | Fail | 0.1725 |
| 32 | Advant | 8 | 90 | 0.1 | 3 | Pass | 101.0 | 1.9 | 3 | Pass | 0.2416 |
| 33 | Advant | 8 | 92 | 1.8 | 6 | Pass | 97.0 | 3.0 | 10 | Pass | 0.1421 |
Figure 1.
Comparison of assay for the samples collected in China and Myanmar. Two vertical dashed lines show the acceptance criteria of 95.0–105.0% assay. This figure appears in color at www.ajtmh.org.
Figure 2.
Comparison of dissolution rate (%) for the samples collected in China and Myanmar. The vertical dashed line shows the acceptance criteria should be more than 75% dissolution rate at 45-minute sampling time. This figure appears in color at www.ajtmh.org.
Handheld Raman spectroscopy and PCA.
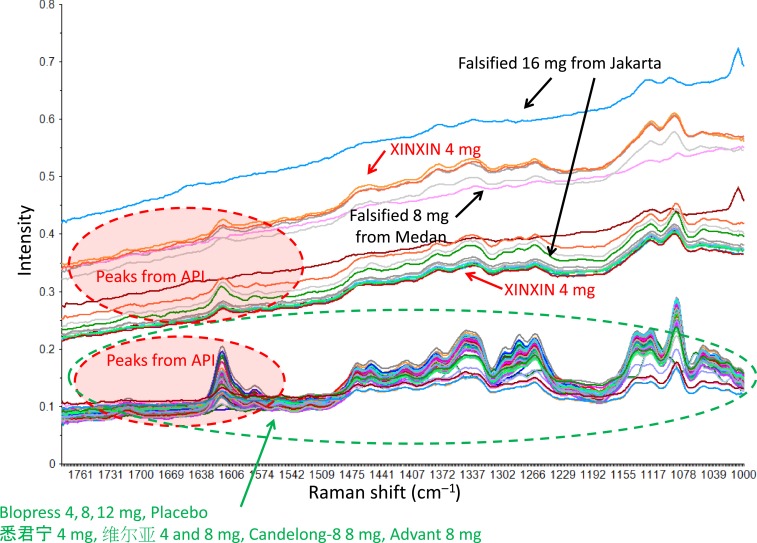
Raman spectra obtained with the handheld instrument are shown in Figure 3. The spectral features are mainly due to the API and the excipients, including lactose monohydrate. The Raman spectra of the falsified products and products stated as XINXIN showed a distinctive upward slop of the baseline toward high wavenumber. The API peak intensity in this region was reported to increase linearly with the increase in API content in the tablets,34,50 and a similar result was also obtained in this study. These relationships of the quantitation between the API peak intensity and the assay of the API in tablet were also confirmed in not only the weight measurement of API versus the peak intensity of Raman spectra but also the relationship between the weight measurement of API versus the peak intensity of X-ray diffraction measurement.50
Figure 3.
Raman spectra of candesartan cilexcetil tablets and the falsified products (before multiplicative scattering correlation preprocessing of Raman spectra). This figure appears in color at www.ajtmh.org.
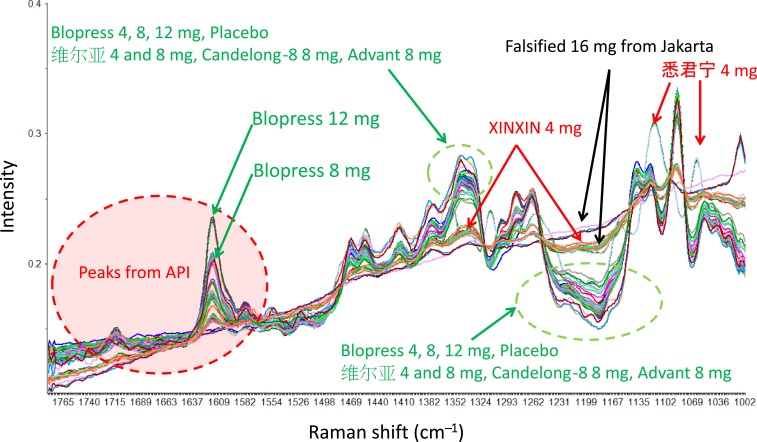
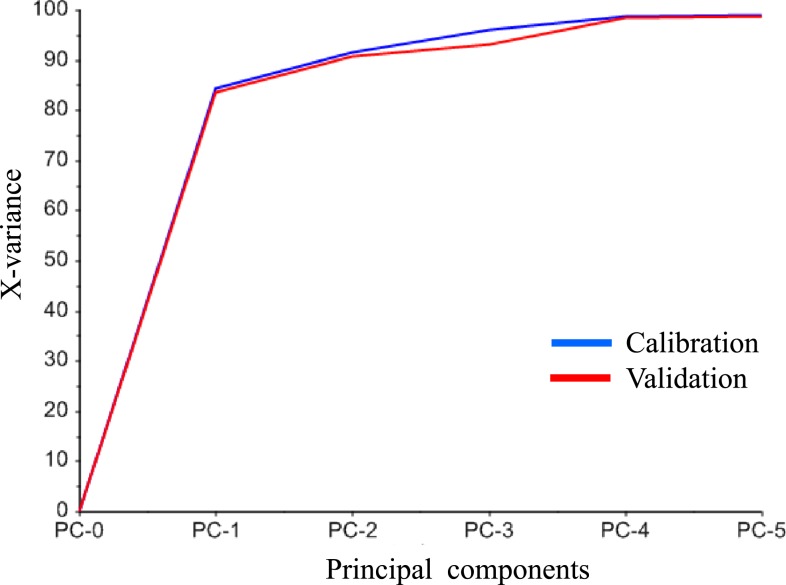
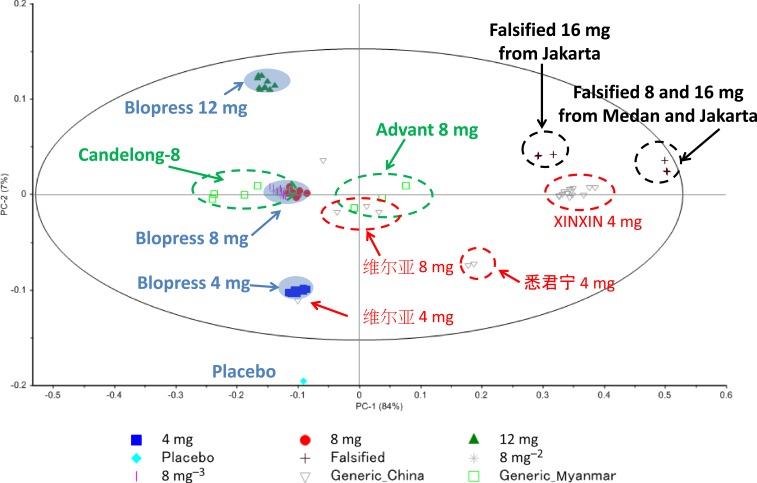
These Raman spectra were conducted preprocessing and subjected to PCA to investigate the similarity of chemical components among samples. Figure 4 shows the spectra after the preprocessing of SG method for smoothing and MSC method for baseline correction for the Raman spectra. The calibration result and the cross-validation result in the PCA model were compared as shown in Figure 5. The result suggested that the difference among the samples can be clarified by using the two principal components of PC1 and PC2, and the intensity change of Raman spectrum can be sufficiently expressed by PC1 and PC2. Therefore, the score plot was shown with the score of the PC1 and PC2 on the horizontal axis and the vertical axis and the vertical axis, respectively, for each tablet as shown in Figure 6. Data set of the Raman spectra in the range of 1780–1700 cm−1, which includes peaks from the API and main excipient6s, showed the intuitive interpretation score plot in the PCA result. Tablets collected in Myanmar were distributed around authentic Blopress tablets in the score plot, suggesting that the similar excipients were used in both cases. On the other hand, the tablets collected in China showed a wide distribution on the score plot, suggesting that different excipients were used by different manufacturers. Notably, the tablets stated as XINXIN were placed very far from the other tablets and there was a high positive correlation in PC1, and the falsified products collected in Indonesia were located similarly in the plot. The falsified products included the API but clearly insufficient assay of the dose displayed on the package (16 mg), as judged from both the Raman spectra and the PC2 correlation compared with that of the authentic products. This result was in agreement with the result of the additional assay measurement (assay: about 60%) obtained using HPLC. In addition, both SFs contained almost the same amount of API, despite being labeled on the packages as having 8 mg and 16 mg, respectively, suggesting that these were falsified products with poor quality control.
Figure 4.
Raman spectra of candesartan cilexetil tablets and the falsified products (after multiplicative scattering correlation preprocessing of Raman spectra). This figure appears in color at www.ajtmh.org.
Figure 5.
Comparison between the calibration model and validation result in principal component analysis model. This figure appears in color at www.ajtmh.org.
Figure 6.
Principal component analysis score plot derived from the Raman spectra of candesartan cilexetil tablets, including the falsified tablets, collected in China, Indonesia, Japan, and Myanmar. This figure appears in color at www.ajtmh.org.
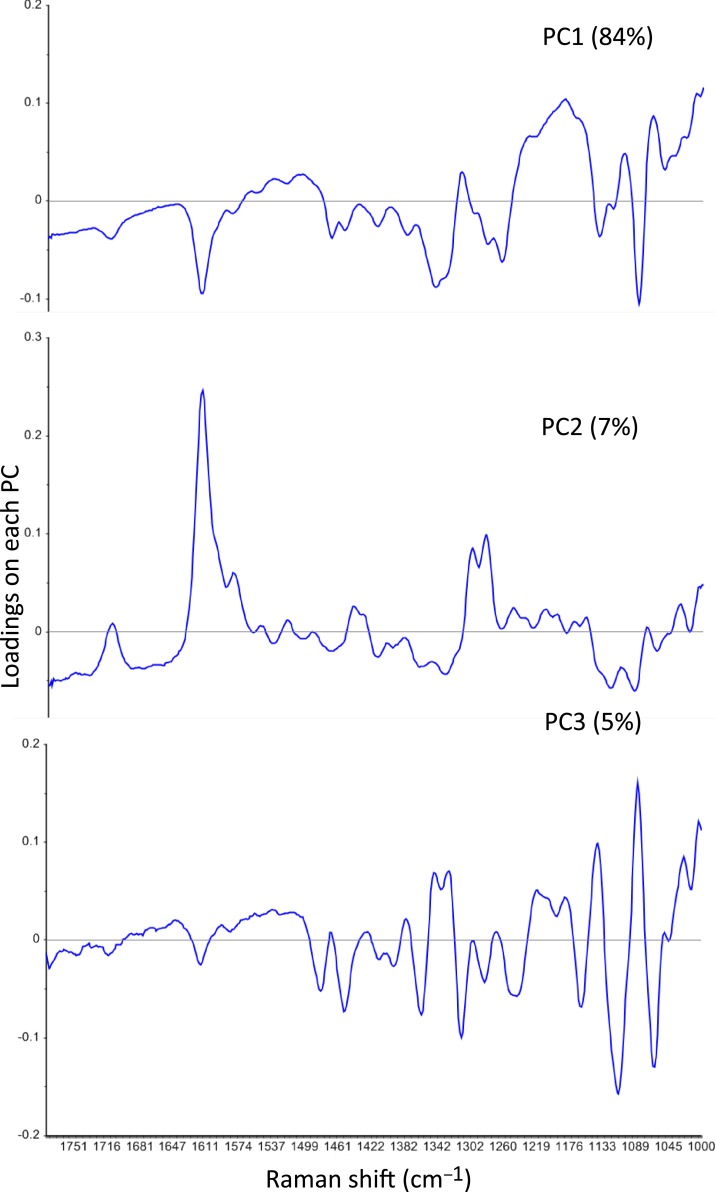
Figure 7 shows the loading of each principal component (PC) in the calculated PCA model. The contribution rates were 84% of PC1, 7% of PC2, and 5% of PC3. PC2 was shown as a component that extracted the characteristics of the signal derived from API, whereas PC1 showed the characteristics of the excipients of the lactose and other excipients in the wave number region of 1200–1000 cm−1. PC3 appeared to be mainly due to lactose factor.
Figure 7.
Loading on PC 1, PC 2, and PC 3 in the principal component analysis model calculated by using Raman spectra of candesartan cilexetil tablets. This figure appears in color at www.ajtmh.org.
DISCUSSION
Candesartan cilexetil tablets distributed in China, Indonesia, and Myanmar were made by various manufacturers and contained different kinds of excipients. Testing identified a number of samples with unacceptable API contents above or below the criteria limit of the Japanese pharmacopeia, and others with excessive dissolution delay. The failed samples that did not meet the criteria were all located far from the center in PCA score plot. Principal component analysis was also very effective in distinguishing different excipients, which appeared in different regions of the score plot. Principal component analysis result was able to explain all spectra clearly with two components, i.e., the medicinal ingredient and the excipient, including lactose. The PCA result decomposed spectrum reflected the elements of pure Raman spectrum on PC2 without interference by background of strong florescence substances. In this study, with an appropriate spectral preprocessing and PCA combination, even in market research using a large amount of Raman spectrum of various kinds of tablets, including some unknown excipients, the elements of the API and the kinds of the excipients are clearly extracted, and the similarity and correlation are clearly visualized.
A key feature of the present work was the use of the MSC method for Raman signal preprocessing. This method proved to be more effective than other commonly used methods, such as the second derivative and standard normal variate methods, for extracting the desired signals from the strong fluorescence background. It was found how to extract the chemical information itself from the spectra of the spectroscope, not the experimental devices and methods, is a significant powerful and effective solution for detecting SFs. These results suggest that the handheld Raman deice we used could be a useful tool to detect SFs in the field, despite its relatively low sensitivity and low resolution.
In conclusion, the combination of pharmacopeial quality control tests and PCA score plots calculated from Raman spectra proved to be a very effective methodology for detecting SFs. Application of this approach to candesartan cilexetil tablets collected in several Asian countries uncovered a number of examples of out-of-specification content and inadequate dissolution. The handheld Raman device is expected to be useful in field surveys to detect SFs. Principal component analysis of that Raman data clarify the difference in chemical properties between good quality products and SFs that circulate in the Asian market.
Acknowledgments:
We are grateful to the Japan Pharmaceutical Manufacturers Association, Myanmar FDA, and Takeda Pharmaceutical Company, Ltd. for supporting this research. We also thank Analytical Development, Pharmaceutical Sciences, Takeda Pharmaceutical Company, Ltd. for advice regarding the analytical technologies. We also thank William Bramstedt, Yasutaka Igari, and Kenichi Shofuda for their help with global collaboration for this study. We would also like to acknowledge Theingi Zin for the support of the grand design for our survey. We also thank Hideyuki Shinzawa and Kaoru Sumitomo for advice on multivariate analysis, and Yoshihiro Uchiyama for technical advice regarding the formulation designs and the spectroscopy.
REFERENCES
- 1.World Health Organization , 2017. Substandard and Falsified (SF) Medical Products. Available at: http://www.who.int/medicines/regulation/ssffc/en/. Accessed June 22, 2017.
- 2.Hall KA, Newton PN, Green MD, De Veij M, Vandenaabele P, Pizzanelli D, Mayfong M, Dondorp A, Fernández F, 2006. Characterization of counterfeit artesunate antimalarial tablets from southeast Asia. Am J Trop Med Hyg 75: 804–811. [PubMed] [Google Scholar]
- 3.Security PG. A Serious Threat to Patient Safety, Counterfeit Pharmaceuticals. Available at: http://www.pfizer.com/files/products/CounterfeitBrochure.pdf. Accessed May 13, 2016.
- 4.de Peinder P, Vredenbregt MJ, Visser T, de Kaste D, 2008. Detection of Lipitor® counterfeits: a comparison of NIR and Raman spectroscopy in combination with chemomertrics. J Pharm Biomed Anal 47: 688–694. [DOI] [PubMed] [Google Scholar]
- 5.Lopes MB, Wolff J-C, 2009. Investigation into classification/sourcing of suspect counterfeit Heptodin™ tablets by near infrared chemical imaging. Anal Chim Acta 633: 149–155. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs S, Hawes SE, Maley SN, Mosites E, Wong K, Stergachis A, 2014. Technologies for detecting falsified and substandard drugs in low and middle-income countries. PLoS One 9: e90601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowell FE, Maghirang EB, Fernandez FM, Newton PN, Green MD, 2008. Detecting counterfeit antimalarial tablets by near-infrared spectroscopy. J Pharm Biomed Anal 48: 1011–1014. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri N, et al. 2014. Evaluation of a new handheld instrument for the detection of counterfeit artesunate by visual fluorescence comparison. Am J Trop Med Hyg 91: 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzgrabe U, Malet-Martino M, 2011. Analytical challenges in drug counterfeiting and falsification-The NMR approach. J Pharm Biomed Anal 55: 679–687. [DOI] [PubMed] [Google Scholar]
- 10.Puchert T, Lochmann D, Menezes JC, Reich G, 2010. Near-infrared chemical imaging (NIR-CI) for counterfeit drug identification—a four-stage concept with a novel approach of data processing (linear image signature). J Pharm Biomed Anal 51: 138–145. [DOI] [PubMed] [Google Scholar]
- 11.Rodionova OYe, Pomerantsev AL, 2010. NIR-based approach to counterfeit-drug detection. TrAC Trends Analyt Chem 29: 795–803. [Google Scholar]
- 12.Reviere H, Guinot P, Chauvey N, Brenier C, 2017. Fighting falsified medicines: the analytical approach. J Pharm Biomed Anal 142: 286–306. [DOI] [PubMed] [Google Scholar]
- 13.Pharmaceutical Security Institute , 2016. Incident-Regions of the World. Available at: http://www.psi-inc.org/geographicDistributions.cfm. Accessed June 19, 2016.
- 14.Banerjee Y, 2017. Counterfeit and substandard drugs in sub-Saharan Africa may pose a major hurdle to H3Africa’s initiative to study genetics of kidney disease progression. Kidney Int 91: 252–253. [DOI] [PubMed] [Google Scholar]
- 15.Antignac M, et al. 2017. Fighting fake medicines: first quality evaluation of cardiac drugs in Africa. Int J Cardiol 243: 523–528. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization , 2016. SSFFC Medical Products. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/mediacentre/factsheets/fs275/en/. Accessed June 19, 2016.
- 17.Khan MH, Okumura J, Sovannarith T, Nivanna N, Akazawa M, Kimura K, 2010. Prevalence of counterfeit anthelminthic medicines: a cross-sectional survey in Cambodia. Trop Med Int Health 15: 639–644. [DOI] [PubMed] [Google Scholar]
- 18.Khan MH, Okumura J, Sovannarith T, Nivanna N, Nagai H, Tara M, Yoshida N, Akazawa M, Tanimoto T, Kimura K, 2011. Counterfeit medicines in Cambodia—possible causes. Pharm Res 28: 484–489. [DOI] [PubMed] [Google Scholar]
- 19.Hoellein L, Holzgrabe U, 2014. Development of simplified HPLC methods for the detection of counterfeit antimalarials in resource-restraint environments. J Pharm Biomed Anal 98: 434–445. [DOI] [PubMed] [Google Scholar]
- 20.Grech J, Robertson J, Thomas J, Cooper G, Naunton M, Kelly T, 2018. An empirical review of antimalarial quality field surveys: the importance of characterising outcomes. J Pharm Biomed Anal 147: 612–623. [DOI] [PubMed] [Google Scholar]
- 21.Been F, Yves RKD, Esseiva P, Margot P, 2011. Profiling of counterfeit medicines by vibrational spectroscopy. Forensic Sci Int 211: 83–100. [DOI] [PubMed] [Google Scholar]
- 22.Dégardin K, Roggo Y, Margot P, 2014. Understanding and fighting the medicine counterfeit market. J Pharm Biomed Anal 87: 167–175. [DOI] [PubMed] [Google Scholar]
- 23. ICH Harmonised Tripartite Guideline Q6A , 1999. Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products Available at: https://www.pmda.go.jp/files/000156754.pdf. Accessed June 22, 2017.
- 24.Höllein L, Kaale E, Mwalwisi YH, Schulze MH, Holzgrabe U, 2016. Routine quality control of medicines in developing countries: analytical challenges, regulatory infrastructures and the prevalence of counterfeit medicines in Tanzania. TrAC Trends Anal Chem 72: 60–70. [Google Scholar]
- 25.Custers D, Krakowska B, De Beer JO, Coureselle P, Daszykowski M, Apers S, Deconinck E, 2016. Chromatographic impurity fingerprinting of genuine and counterfeit Cialis® as a means to compare the discriminating ability of PDA and MS detection. Talanta 146: 540–548. [DOI] [PubMed] [Google Scholar]
- 26.Plonka M, Walorczyk S, Miszczyk M, 2016. Chromatographic methods for the determination of active substances and characterization of their impurities in pesticide formulations. TrAC Trends in Analytical Chemistry 85: 67–80. [Google Scholar]
- 27.Custers D, Cauwenbergh T, Bothy JL, Courselle P, De Beer JO, Apers S, Deconinck E, 2015. ATR-FTIR spectroscopy and chemometrics: an interesting tool to discriminate and characterize counterfeit. J Pharm Biomed Anal 112: 181–189. [DOI] [PubMed] [Google Scholar]
- 28.Stewart MW, Narayanan R, Gupta V, Rosenfeld PJ, Martin DF, Chakravarthy U, 2016. Counterfeit Avastin in India: punish the criminals, not the patients. Am J Ophthalmol 170: 228–231. [DOI] [PubMed] [Google Scholar]
- 29.Conway J, Bero L, Ondari C, Wasan KM, 2013. Review of the quality of pediatric medications in developing countries. J Pharm Sci 102: 1419–1433. [DOI] [PubMed] [Google Scholar]
- 30.Ministry of Health, Labour and Welfare , 2016. Candesartan cilexetil tablets. Drug Monograph of the Japanese Pharmacopea, 17th edition. Tokyo, Japan: Ministry of Health, Labour and Welfare, 663–665.
- 31.Habyalimana V, Mbinze JK, Yemoa AL, Waffo C, Diallo T, Tshilombo NK, Ntokamunda JK, Lebrun P, Hubert P, Marini RD, 2017. Application of design space optimization strategy to the development of LC methods for simultaneous analysis of 18 antiretroviral medicines and 4 major excipients used in various pharmaceutical formulations. J Pharm Biomed Anal 139: 8–21. [DOI] [PubMed] [Google Scholar]
- 32.Abdellah A, Noordin MI, Wan AWI, 2015. Importance and globalization status of good manufacturing practice (GMP) requirements for pharmaceutical excipients. Saudi Pharm J 23: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Arieta A, 2014. Interactions between active pharmaceutical ingredients and excipients affecting bioavailability: impact on bioequivalence. Eur J Pharm Sci 65: 89–97. [DOI] [PubMed] [Google Scholar]
- 34.Kakio T, Yoshida N, Macha S, Moriguchi K, Hiroshima T, Ikeda Y, Tsuboi H, Kimura K, 2017. Classification and visualization of physical and chemical properties of falsified medicines with handheld Raman spectroscopy and x-ray computed tomography. Am J Trop Med Hyg 97: 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinzawa H, Hashimoto K, Sato H, Kanematsu W, Noda I, 2014. Multiple-perturbation two-dimensional (2D) correlation analysis for spectroscopic imaging data. J Mol Struct 1069: 176–182. [Google Scholar]
- 36.Sacre P-Y, De Bleye C, Chavez P-F, Netchacovitch L, Hubert Ph, Ziemons E, 2014. Data processing of vibrational chemical imaging for pharmaceutical applications. J Pharm Biomed Anal 101: 123–140. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe A, Morita S, Kokot S, Matsubara M, Fukai K, Ozaki Y, 2006. Drying process of microcrystalline cellulose studied by attenuated total reflection IR spectroscopy with two-dimensional correlation spectroscopy and principal component analysis. J Mol Struct 799: 102–110. [Google Scholar]
- 38.Franco D, et al. 2017. Raman spectroscopy differentiates between sensitive and resistant multiple myeloma cell lines. Spectrochim Acta A Mol Biomol Spectrosc 187: 15–22. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa T, 2007. Quantitative Analytical Techniques of Spectra, 2nd edition. Tokyo, Japan: Kodansya. [Google Scholar]
- 40.Shimada T, Hasegawa T, 2017. Determination of equilibrium structures of bromothymol blue revealed by using quantum chemistry with an aid of multivariate analysis of electronic absorption spectra. Spectrochim Acta A Mol Biomol Spectrosc 185: 104–110. [DOI] [PubMed] [Google Scholar]
- 41.Shimaoka T, Hasegawa T, 2016. Molecular structural analysis of hydrated ethylene glycol accounting for the antifreeze effect by using infrared attenuated total reflection spectroscopy. J Mol Liq 223: 621–627. [Google Scholar]
- 42.Hasegawa T, 2001. Detection of minute chemical signals by principal component analysis. TrAC Trends Anal Chem 20: 53–64. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa T, Nishijo J, Umemura J, 2000. Separation of Raman spectra from fluorescence emission background by principal component analysis. Chem Phys Lett 317: 642–646. [Google Scholar]
- 44.Ministry of Health, Labour and Welfare , 2016. “1.Conent uniformity” in <6.02>. Drug Monograph of the Japanese Pharmacopea, 17th edition. Tokyo, Japan: Ministry of Health, Labour and Welfare, B-604–B-613.
- 45.Lohumi S, Kim MS, Qin J, Cho B-K, 2017. Raman imaging from microscopy to macroscopy: quality and safety control of biological materials. TrAC Trends Analyt Chem 93: 183–198. [Google Scholar]
- 46.Xie Y, Yang L, Sun X, Wu D, Chen Q, Zeng Y, Liu G, 2016. An auto-adaptive background subtraction method for Raman spectra. Spectrochim Acta A Mol Biomol Spectrosc 161: 58–63. [DOI] [PubMed] [Google Scholar]
- 47.Ritthiruangdej P, Ritthiron R, Shinzawa H, Ozaki Y, 2011. Non-destructive and rapid analysis of chemical compositions in Thai steamed pork sausages by near-infrared spectroscopy. Food Chem 129: 684–692. [DOI] [PubMed] [Google Scholar]
- 48.Næs T, Isaksson T, Fearn T, Davies T, 2002. A User-Friendly Guide to Multivariate Calibration and Classification. Chichester, United Kingdom: NIR Publications. [Google Scholar]
- 49.Phatak A, 2004. Book review of a user-friendly guide to multivariate calibration and classification. Chemom Intell Lab Syst 71: 79–81. [Google Scholar]
- 50.Kakio T, Hiroshima T, Ikeda Y, 2014. Development of quantitative analysis for Polymorph of drug substances in pharmaceutical oral dosage forms by XRPD and Raman spectroscopy. J Pharm Machinery Eng 23: 140–146. [Google Scholar]