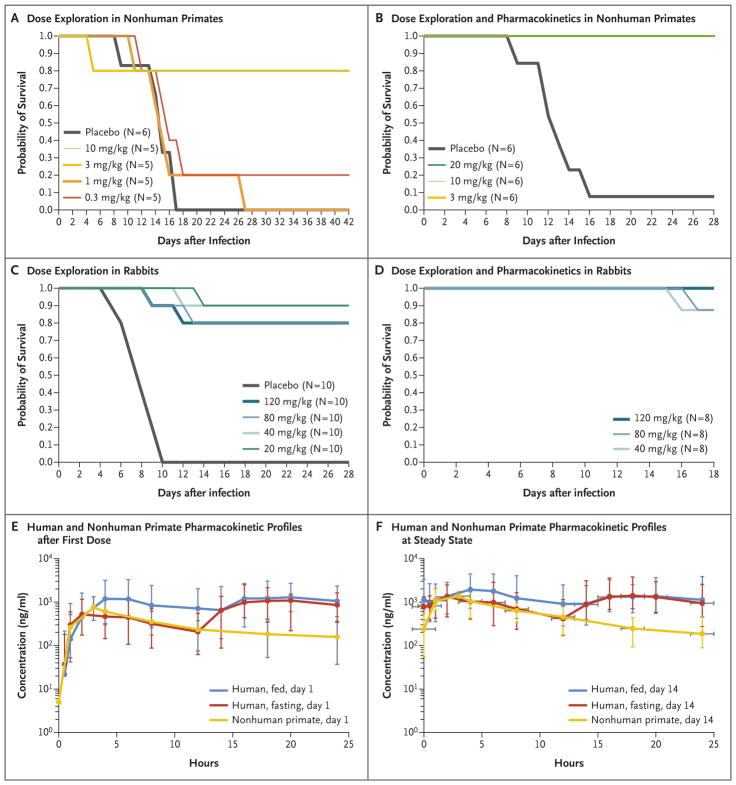
Figure 1. Efficacy and Pharmacokinetic Profiles of Tecovirimat in Animal Models.
On day 0, nonhuman primates (Panels A and B) and rabbits (Panels C and D) were infected with a lethal dose of monkeypox virus or rabbitpox virus, respectively. Survival was monitored for 18 to 42 days after infection, indicated on the horizontal axis labels. Tecovirimat was administered by oral gavage at doses ranging from 0.3 to 20 mg per kilogram of body weight in nonhuman primates and from 20 to 120 mg per kilogram in rabbits. Tecovirimat was administered for 14 consecutive daily doses starting on day 4 after infection, after the onset of clinical signs (pock lesions in nonhuman primates and fever and viremia in rabbits) in each study. Comparison of the exposures required for efficacy in rabbits and nonhuman primates showed that the nonhuman primate was the more conservative model, with higher exposures required for full effectiveness. Therefore, the exposure profiles of tecovirimat in plasma in nonhuman primates and humans were compared after the first dose (Panel E) and after the last dose at steady state (Panel F) to evaluate whether exposures in humans exceeded those in nonhuman primates, providing a reasonable expectation of efficacy in humans.