Abstract
Background
There are numerous definitions of multimorbidity. None systematically examines specific comorbidity combinations accounting for multiple testing when exploring large datasets.
Objectives
Develop and validate a list of all single, double, and triple comorbidity combinations, with each individual Qualifying Comorbidity Set (QCS) at least doubling the odds of mortality versus its reference population. Patients with at least one QCS were defined as having multimorbidity.
Research Design
Cohort-based study with a matching validation study.
Subjects
All fee-for-service Medicare patients between age 65 and 85 without dementia or metastatic solid tumors undergoing general surgery in 2009–2010, and an additional 2011–2013 dataset.
Measures
30-day all-location mortality
Results
There were 576 qualifying comorbidity sets (2 singles, 63 doubles and 511 triples), each set at least doubling the odds of dying. In 2011, 36% of eligible patients had multimorbidity. As a group, multimorbid patients (mortality rate = 7.0%) had a mortality Mantel-Haenszel OR = 1.90 (1.77, 2.04) versus a reference that included both multimorbid and non-multimorbid patients (mortality rate = 3.3%), and Mantel-Haenszel OR = 3.72 (3.51, 3.94) versus only non-multimorbid patients (mortality rate = 1.6%). When matching 3,151 pairs of multimorbid patients from low-volume hospitals to similar patients in high-volume hospitals, the mortality rates were 6.7% versus 5.2%, respectively (P = 0.006).
Conclusions
A list of QCSs identified a third of older patients undergoing general surgery that had greatly elevated mortality. These sets can be used to identify vulnerable patients and the specific combinations of comorbidities that make them susceptible to poor outcomes.
Keywords: Multimorbidity, Medicare, Mortality, General Surgery
INTRODUCTION
There are a number of definitions of multimorbidity that have been advocated by various groups.1–6 Some use comorbidity indexes to study multimorbidity,7–9 while others define multimorbidity as a list of 1 or more conditions,10–19 and still others define it as 2 or more conditions.13,20–31 Some are interested in using such definitions to directly influence care,2,5,11,15, 19–21,28,29,32–35 while others are interested in measuring or benchmarking quality of care in these more challenging patients.2,5,7,9,10,12,16–18,23–26,30,36 Multimorbidity lists utilize various datasets, some dependent on claims data,7,22,23,33,37–42 while others incorporate additional clinical items not generally found in administrative data.6,8,9,11,12,14,16,17,23,26,29,31–33,40
In this study, we present a new definition for identifying multimorbid patients in general surgery and develop a list of qualifying comorbidity sets (QCSs) that operationalize that definition. The goal of this project was to develop a list of comorbidity sets that could be used to define a group of patients with multimorbidity. Such a list would be preferable to simply defining patients with increased risk through a multivariable model for two reasons: First, we believe clinicians want to be able to identify specific patients with multimorbidity based on specific reasons for that classification. Knowing that a patient is high-risk from a model does not convey the same depth of information as knowing that the patient is high-risk with multimorbidity because of specific comorbidities. Second, we want to ultimately match these specific comorbidity combinations in order to aid quality improvement actions for specific types of patients. The list can also inform surgeons of patients that have especially high risk of 30-day mortality when weighing whether to recommend a proposed procedure. Finally, the new methodology and resulting definition that we present herein can be used as a template to develop lists of comorbidity sets for other medical and surgical patients.
METHODS
Conceptual Model and Design
To develop a claims-based list of qualifying comorbidity sets that define mutimorbidity, we start with a number of desired properties: (1) The list should provide an estimate of the increased odds of mortality relative to a general population undergoing that same surgery. In so doing, we want a typical representative population of patients undergoing general surgery to serve as the control group that informs us as to how much the comorbidity set of interest elevates the risk of death above the baseline average; (2) Each elevated odds ratio associated with a comorbidity set must have a statistically definable confidence interval and p-value. Here we will define this elevated risk as the lower bound of a 95% confidence interval; (3) The list should include the more common comorbidity combinations that represent at least 0.5% of patients in the population, because the list should be practical for use in regression and matching applications and yield adequate data to support refined analyses; (4) The list development process should exclude patients with some conditions that are already high-risk or may influence the attempt of caregivers and family to address life-threatening complications. For this analysis, we excluded patients with metastatic solid cancer or dementias, and also excluded patients aged 85 and older for similar reasons. We therefore concentrate on comorbidity combinations that increase risk among patients who lack these excluding characteristics, but would suggest that when utilizing our list, these other categories could be explored depending on the purpose of the application (as will be illustrated).
Patient Population
We were granted access via the Centers for Medicare & Medicaid Services (CMS) Virtual Research Data Center (VRDC) to administrative claims data for older Medicare beneficiaries who were admitted to acute care hospitals in the United States for general surgery in 2009–2013. The VRDC hosted each patient's beneficiary summary file (Base, Chronic Conditions, Cost & Utilization, and Other Chronic or Potentially Disabling Conditions segments) and administrative claims (Inpatient, Outpatient, Carrier/Part B, Hospice, and Home Health Agency files). We leveraged these files for a comprehensive assessment of disease burden in the 12 months prior to each patient’s index hospitalization for general surgery.
Admissions in 2009–2010 were used for the development of comorbidity sets. We applied exclusion steps that would permit complete ascertainment of comorbid conditions in the period prior to admission. Specifically, we excluded hospitalizations where the patient was under 66 years old at admission, the patient had evidence of hospice care, or the patient lacked complete fee-for-service Medicare in the 12 months prior to admission, the month of admission, or the month after admission. We also restricted the sample to the 50 U.S. states and the District of Columbia. Finally, if a patient had multiple qualifying admissions for general surgery in 2009–2010, we chose a random one.
To illustrate one use of the newly developed comorbidity sets, in an additional dataset of 2011 admissions, we asked whether multimorbid patients defined as having at least one QCS who were admitted to hospitals with Medicare general surgery volume in the upper 10th percentile of hospitals in the U.S. (a characteristic generally associated with better outcomes43) would have lower 30-day mortality than multimorbid patients admitted to hospitals in the lower third.
Finally, we assessed the replicability of our algorithm by analyzing the same surgical population described above for the additional years 2012 and 2013.
Outcomes
The outcome used to assess risk was all-cause, all-location mortality within 30 days of admission.
Statistical Methods
Defining Qualifying Comorbidity Sets
We developed our methodology using a random 20% sample of unique eligible patients admitted for one of the general surgery procedures listed in Table 1 from 2009 to 2010 (excluding patients with metastatic solid cancer, Alzheimer's Disease and related disorders, and age 85 or older for this study). This 20% sample was not used in subsequent analyses, which refer to the complementary 80% random sample.
Table 1. ICD-9 Codes for General Surgery Procedures of Interest.
| Adrenal Procedures | 0722; 0729; 073 |
| Appendectomy | 4701; 4709 |
| Bowel Anastomoses | 4590; 4591; 4592; 4593; 4594; 4595 |
| Bowel Procedures, Other | 4673; 4674; 4675; 4679 |
| Breast Procedures | 8522; 8523; 8541; 8542; 8543; 8544; 8545; 8546; 8547; 8548 |
| Esophageal Procedures | 4240; 4241; 4242; 427 |
| Femoral Hernia Procedures | 5321; 5329 |
| Gallbladder Procedures | 5122; 5123; 5124; 5132; 5136; 5137; 5141; 5151 |
| Incisional and Abdominal Hernias | 537; 5361; 5369; 5372; 5375 |
| Inguinal Hernia Procedures | 1711; 1712; 1713; 1721; 1722; 1723; 1724; 5300; 5301; 5302; 5303; 5304; 5305; 5310; 5311; 5312; 5313; 5314; 5315; 5316; 5317 |
| Large Bowel Resection | 1731; 1732; 1733; 1734; 1735; 1736; 1739; 4571; 4572; 4573; 4574; 4575; 4576; 4579; 458; 4581; 4582; 4583 |
| Liver Procedures | 5022; 5029; 503; 5059 |
| Lysis of Adhesions | 5451; 5459 |
| Ostomy Procedures | 4601; 4603; 4610; 4611; 4613; 4620; 4621; 4622; 4623; 4639; 4642; 4651; 4652 |
| Pancreatic Procedures | 5252; 5259; 526; 527 |
| Parathyroidectomy | 0681; 0689 |
| PD Access Procedure | 5493 |
| Rectal Procedures | 4849; 485; 4850; 4851; 4852; 4859; 4862; 4863; 4869; 4875; 4876; 4879 |
| Repair of Vaginal Fistulas | 7072; 7073; 7074; 7075 |
| Small Bowel Resection | 4561; 4562; 4563 |
| Splenectomy | 415 |
| Stomach Procedures | 4342; 435; 436; 437; 4389; 4399; 4429; 4438; 4439; 4466; 4467; 4469; 4495 |
| Thyroid Procedures | 062; 0631; 0639; 064; 0650; 0651; 0652 |
| Ulcer Surgery | 4441; 4442 |
| Umbilical Hernia Procedures | 5341; 5349 |
| Ventral Hernia Repair | 5351; 5359 |
The algorithm for constructing the list of qualifying comorbidity sets was as follows:
Step 1: Construct Candidate Sets of Comorbidities: For this study, we considered the 50,183 possible comorbidity combinations that can be formed from 67 candidate comorbidities. Our study team formed the list of 67 comorbidities by taking the union of comorbidities derived from extensive lists of comorbidities from the AHRQ chronic conditions dataset, the AHRQ Elixhauser comorbidities, the Hospital Compare comorbidities, and a list of surgical comorbidities we have used in our previous work (see Supplemental Digital Content Section 1). We considered all triples of three comorbidities, all pairs of two comorbidities, and all individual comorbidities. Comorbidities were assigned to patients if they appeared in a 12-month lookback or were present on admission for the index hospitalization. Some related comorbidities were classified into mutually exclusive severity levels. If a patient had both more and less severe manifestations of the same condition, the patient was only classified as having the more severe manifestation. Specifically, "chronic renal failure with dialysis" (CRFD) was defined as mutually exclusive from "renal dysfunction," such that if a patient had a history of CRFD and renal dysfunction in the past year, then the patient was recorded as having CRFD, but not renal dysfunction. Similarly, if a patient had diagnoses for both moderate/severe diabetes and mild diabetes in the past year, the patient was recorded as having moderate/severe diabetes, but not mild diabetes.
Step 2: Cross-screening of comorbidity sets for elevated risk: If 50,183 true null hypotheses are tested at the 0.05 level, it is expected 0.05×50,183 = 2,905 of them will be falsely rejected. To avoid identifying 2,905 nonsense comorbidity sets by chance, we used the cross-screening methodology of Zhao, Small, and Rosenbaum,44 an alternative to the Bonferroni adjustment, which would require comorbidity sets to be significant at 0.05/50,183. In cross-screening, the data are split in half at random. The first random half suggests a limited number of comorbidity sets to be validated in the second random half. The second random half suggests a limited number of comorbidity sets to be validated in the first random half. The validated comorbidity sets are those that validate in at least one half. Both halves are used to screen, and both halves are used to validate. A validated comorbidity set must look promising in one half and pass a formal statistical test in the other half, so it must pass two tests. Because a limited number of comorbidity sets seek validation on the second half, the Bonferroni adjustment used in the validation sample divides by a small fraction of the 50,183 potential hypotheses. For detailed discussion of cross-screening, its properties and limitations, see Zhao, Small, and Rosenbaum.44 Cross-screening strongly controls the family-wise error rate: the chance that it falsely rejects one or more true null hypotheses is at most 0.05, despite having screened 50,183 combinations of comorbidities. It is very unlikely that even one of our comorbidity sets fails to achieve its stated property of double the conditional odds of death relative to the reference population.
Step 3: Specifics of implementing cross-screening: We wanted comorbidity sets that were associated with at least a doubling of the odds of 30-day mortality compared to the general population undergoing the same procedure because a 2-fold increase in the odds of 30-day mortality was clinically important and produced a group of multimorbid patients comprising about a third of eligible patients when tested in our initial 20% sample. This seemed desirable since too large a number would reduce the usefulness of the categorization of multimorbidity when focusing on quality improvement, and too few would create sample size problems when examining subgroups. Of note, we excluded patients with evidence of dialysis from the reference group, because from a clinical perspective, this group disproportionately increased the reference group’s risk in our initial 20% sample. We used the Mantel-Haenszel test45 in each random half of the data to screen comorbidity sets that could possibly occur subject to the mutually exclusive severity levels described earlier, and which had a prevalence of at least 0.5%. These requirements yielded 6,559 possible combinations. The Mantel-Haenszel test was stratified by the 156 general surgical principal procedures (see Table 1 for details), 2 age groups (≥66 to <75, 75 to <85), and a variable describing whether each patient was in the study population or in the reference group. The resulting 2×2×156 table compared 30-day mortality for patients with a given comorbidity set relative to a representative reference population who had varied comorbidity sets or no comorbidity sets, adjusting for the procedure. In the screening stage of cross-screening, a 99.9% confidence interval was formed, and if this interval was completely above 2, this comorbidity set was referred to the second half-sample for validation by a more stringent standard. In cross-screening, if K comorbidity sets were selected by this screening step using the first half-sample, then just these K comorbidity sets were tested using the second half-sample, testing the null hypothesis of an odds ratio ≤ 2 at level 0.05/(2K). Also, if K’ comorbidity sets were selected by this screening step using the second half-sample, then just these K’ comorbidity sets were tested using the first half-sample, testing the null hypothesis of an odds ratio ≤ 2 at level 0.05/(2K’). Any comorbidity set that validated in either half was included in our list of qualifying comorbidity sets. The chance that even one comorbidity set with an odds ratio ≤ 2 incorrectly made it onto our list is at most 0.05: with 95% confidence, every qualifying comorbidity set on our list has an odds ratio >2 relative to the reference group. All statistical tests were completed using SAS v 9.4.46
The 2011 Matching Application Study
We explored how patients with our new definition of multimorbidity fared at hospitals in the upper decile of general surgery volume relative to the lower third. Hospital volumes were calculated using all available Medicare inpatient bills. Index admissions for matching were screened for data quality in the same manner as was done for 2009 and 2010, and if a patient had multiple general surgery admissions in 2011, a random admission was chosen.
In this analysis, we matched “focal” patients treated at low-volume hospitals to controls treated at high-volume hospitals. We matched exactly for ICD-9 principal procedure and multimorbidity (yes, no) as defined by our newly developed list. Within exact groups, specific QCSs defining multimorbidity were balanced using a refined balance technique47 between the low and high-volume groups; age categories, sex, race, admission type, and individual comorbidities were also included in the balance hierarchy. Other matching variables included a propensity score for admission to a low-volume hospital,48 and a risk score for 30-day mortality (on admission), derived from a model constructed on the initial 20% development data from 2009–10 (see Supplemental Digital Content Section 2 for the model details). We also minimized the total Mahalanobis distance between cases and controls on the matching covariates. Refined balance was implemented using the NETFLOW procedure in SAS,46 but is available more directly in the R package rcbalance.49
Matches were considered balanced if all covariates had standardized differences below 0.2 after matching. Differences in 30-day mortality between matched pairs were tested using the McNemar statistic.50
RESULTS
In Table 2 we describe the 80% sample development dataset (2009–2010) and the 2011 application dataset. The sample sizes were large, and both populations looked very similar in terms of age, sex, and comorbidities.
Table 2. Sample population characteristics.
The Development Dataset represents the 80% random sample of 2009–2010 patients used in the split-sample cross-screening design. The Application dataset respresents the additional dataset used to calculate out-of-sample point estiamtes and 95% confidence intervals for the qualifying comorbidity sets. Both datasets exclude patients aged 85 and older, those with metastatic solid tumors, and Alzheimer’s Disease and related disorders.
| Patient Covariates | Development Dataset (2009–2010) N = 303,236 patients |
Application Dataset (2011) N = 170,575 patients |
|---|---|---|
|
| ||
| Demographics | ||
| Age At Admission (mean) | 74.47 | 74.32 |
| Age 65–69 | 26.5% | 27.6% |
| Age 70–74 | 28.8% | 28.8% |
| Age 75–79 | 24.8% | 24.3% |
| Age 80–84 | 19.9% | 19.2% |
| Sex Male | 43.0% | 43.4% |
| Sex Female | 57.0% | 56.6% |
| Emergent Type | 38.5% | 40.4% |
| Transfer-in | 0.5% | 0.4% |
| Comorbidities | ||
| Hyperlipidemia | 72.3% | 75.0% |
| Arthritis Collagen Vascular | 40.9% | 43.3% |
| AMI/Ischemic Heart | 38.8% | 38.6% |
| Anemia (not Sickle Cell) | 38.5% | 40.2% |
| Cataract | 36.5% | 35.9% |
| COPD | 29.5% | 29.5% |
| Trauma | 26.8% | 27.1% |
| Peripheral Vascular Disease | 25.8% | 26.5% |
| Hypothyroidism | 24.3% | 25.5% |
| Arrhythmia Non-Emergency | 22.9% | 23.8% |
| Renal Dysfunction | 21.8% | 24.2% |
| Valvular Heart Disease | 20.8% | 21.1% |
| Stroke/TIA | 20.0% | 20.3% |
| Heart Failure | 19.9% | 19.8% |
| Diabetes Mod/Severe | 19.5% | 20.4% |
| Smoking or Tobacco Abuse | 18.8% | 24.6% |
| Atrial Fibrillation | 18.0% | 18.9% |
| Diabetes Mild | 16.6% | 17.0% |
| HTN Complicated | 16.2% | 18.5% |
| Osteoporosis | 16.0% | 16.1% |
| Benign Hyperplasia | 15.8% | 17.0% |
| Depression | 14.4% | 16.8% |
| Obesity | 14.2% | 18.9% |
| Glaucoma | 13.7% | 13.7% |
| Weight Loss/Malnutrition | 13.5% | 15.0% |
| Colorectal Cancer | 13.0% | 12.5% |
| Liver Disease | 12.9% | 13.8% |
| Pneumonia | 12.4% | 11.7% |
| Fibromyalgia | 11.8% | 13.7% |
| Asthma | 11.6% | 12.3% |
| Tumor Solid | 10.5% | 10.4% |
| Blood Disorder | 7.8% | 9.4% |
| Breast Cancer | 7.5% | 7.5% |
| Neuro Other | 6.2% | 6.7% |
| Prostate Cancer | 5.7% | 5.8% |
| Coagulopathy | 4.9% | 4.7% |
| Thromboembolic Disease | 4.6% | 4.7% |
| Pulmonary Circulation Disorder | 4.6% | 5.4% |
| Thrombocytopenia | 3.8% | 5.2% |
| Arrhythmia Emergency | 3.4% | 3.4% |
| Lymphoma Leukemia | 2.9% | 2.8% |
| Kidney/Bladder Cancer | 2.8% | 2.9% |
| Vertebral Fracture | 2.2% | 2.2% |
| Female Gynecologic Cancer | 2.1% | 2.2% |
| Seizure | 2.1% | 2.1% |
| Neurodegenerative | 1.7% | 1.6% |
| CRFD | 1.7% | 1.7% |
| Psychosis | 1.7% | 1.7% |
| Hematologic Severe | 1.6% | 1.6% |
| Lung Disease | 1.6% | 1.6% |
| Hip Fracture | 1.5% | 1.4% |
| Paralysis from Stroke | 1.1% | 1.1% |
| Metabolic Developmental Disorders | 0.9% | 1.0% |
| Cardiac Structural | 0.7% | 0.6% |
| Chronic Peptic Ulcer | 0.6% | 0.6% |
| Hyperthyroid/Graves | 0.6% | 0.6% |
| Amputation | 0.6% | 0.7% |
| Paralysis from Trauma | 0.3% | 0.3% |
| Multiple Sclerosis | 0.3% | 0.3% |
| ADHD & Conduct Disorders | 0.2% | 0.2% |
| Brain Cancer | 0.1% | 0.1% |
| HIV/AIDS | 0.1% | 0.1% |
| Male Genital Urinary Cancers | 0.1% | 0.1% |
| Cerebral Palsy | 0.1% | 0.1% |
| Sickle Cell Anemia | 0.1% | 0.0% |
| Mild Cognitive Impairment | 0.0% | 0.0% |
| Autism | 0.0% | 0.0% |
We provide the parsimonious list of the final validated qualifying comorbidity sets in Table 3. There were 576 comorbidity sets that validated in our cross-screening algorithm, and we created the parsimonious list of 113 by first including the singles (2), then the doubles not covered by the singles (33), and then triples not covered by singles or doubles (78). The parsimonious list identifies all multimorbid patients, but would not describe all specific QCSs (see Supplemental Digital Content Section 3 for a complete list of all 576 validated QCSs, and Sections 4 and 5 for the details of the development of the lists). Some important observations can be made. First, two comorbidities each provided a strong signal that such a patient had at least double the odds of dying compared to the reference group. These were a history of chronic renal failure on dialysis ("CRFD") and a history of "Emergency Arrhythmia." Ultimately, our algorithm selected only 25 of the initial 67 candidate comorbidities as elements of the non-duplicative list of 113 qualifying comorbidity sets (see Supplemental Digital Content Section 6 for a description of these 25 comorbidities, and their association with other comorbidities that constitute the QCSs).
Table 3. Odds of Mortality by Qualifying Comorbidity Set.
There were 576 qualifying comorbidity sets (QCSs) that met our statistical criteria in the development dataset (2009–2010), and of these, a parsimonious set of 113 is displayed. This list of 113 QCSs includes all sets of singles (2), doubles (33), and triples (78) without duplicating any set whose components are already on the list as singles or doubles. When applied to a 2011 out-of-sample dataset of 170,575 patients, the multimorbidity definition identified 61,772 unique patients (36.21%). The displayed odds ratios are computed from this same 2011 dataset. The two reference groups are a 20% random sample of 2011 patients (excluding 589 CRFD patients), and a second reference group representing only patients that did not have one of the 576 qualifying comorbidity sets. Note, these data exclude any patients age 85 or older, or with a history of dementia or metastatic solid cancer. We examine the odds of mortality for this final group at the bottom of the table.
| Qualifying Comorbidity Set* | Frequency (%)† | General Reference Group |
Non-MM Reference Group |
|---|---|---|---|
| 1. COPD | Renal Dys | Thrombocytopenia | 1,614 (0.95%) | 4.07 (3.42, 4.86) | 7.64 (6.51, 8.95) |
| 2. CRFD | 2,929 (1.72%) | 4.03 (3.46, 4.69) | 7.81 (6.78, 8.98) |
| 3. Atrial Fib | COPD | Thrombocytopenia | 1,444 (0.85%) | 3.92 (3.26, 4.71) | 7.55 (6.39, 8.92) |
| 4. COPD | HTN Complicated | Thrombocytopenia | 1,450 (0.85%) | 3.83 (3.18, 4.62) | 7.31 (6.17, 8.66) |
| 5. COPD | PVD | Thrombocytopenia | 1,749 (1.03%) | 3.61 (3.04, 4.28) | 6.81 (5.83, 7.96) |
| 6. Atrial Fib | HTN Complicated | Wt Loss/Malnut | 2,609 (1.53%) | 3.61 (3.14, 4.15) | 6.90 (6.09, 7.83) |
| 7. COPD | Arr Non-Emerg | Thrombocytopenia | 1,527 (0.90%) | 3.61 (2.99, 4.36) | 6.62 (5.58, 7.85) |
| 8. Pneumonia | Thrombocytopenia | 2,268 (1.33%) | 3.54 (3.02, 4.14) | 6.48 (5.62, 7.47) |
| 9. HTN Complicated | PVD | Thrombocytopenia | 1,598 (0.94%) | 3.52 (2.93, 4.23) | 6.59 (5.57, 7.79) |
| 10. AMI/Isch Heart | COPD | Thrombocytopenia | 2,273 (1.33%) | 3.45 (2.95, 4.04) | 6.38 (5.53, 7.36) |
| 11. COPD | HTN Complicated | Wt Loss/Malnut | 3,395 (1.99%) | 3.41 (3.00, 3.88) | 6.55 (5.84, 7.35) |
| 12. COPD | Thrombocytopenia | Valvular Heart Disease | 1,479 (0.87%) | 3.38 (2.79, 4.10) | 6.10 (5.11, 7.27) |
| 13. COPD | Renal Dys | Wt Loss/Malnut | 4,233 (2.48%) | 3.37 (2.99, 3.79) | 6.43 (5.80, 7.14) |
| 14. COPD | Neuro Other | Renal Dys | 2,290 (1.34%) | 3.36 (2.86, 3.94) | 6.44 (5.59, 7.43) |
| 15. Atrial Fib | COPD | Wt Loss/Malnut | 3,452 (2.02%) | 3.35 (2.96, 3.80) | 6.55 (5.86, 7.33) |
| 16. Atrial Fib | HTN Complicated | Liver Disease | 1,678 (0.98%) | 3.34 (2.75, 4.05) | 6.30 (5.32, 7.47) |
| 17. Heart Failure | Thrombocytopenia | 3,370 (1.98%) | 3.26 (2.84, 3.75) | 6.05 (5.33, 6.86) |
| 18. Anemia | HTN Complicated | Thrombocytopenia | 2,543 (1.49%) | 3.26 (2.79, 3.81) | 6.16 (5.35, 7.09) |
| 19. COPD | Pulm Circ Disorder | Wt Loss/Malnut | 1,469 (0.86%) | 3.22 (2.68, 3.88) | 6.68 (5.67, 7.86) |
| 20. HTN Complicated | PVD | Wt Loss/Malnut | 3,557 (2.09%) | 3.19 (2.81, 3.62) | 6.23 (5.56, 6.98) |
| 21. Atrial Fib | COPD | HTN Complicated | 4,945 (2.90%) | 3.19 (2.83, 3.59) | 6.42 (5.78, 7.12) |
| 22. Atrial Fib | COPD | PVD | 6,510 (3.82%) | 3.18 (2.86, 3.54) | 6.37 (5.80, 7.00) |
| 23. Pneumonia | Wt Loss/Malnut | 5,767 (3.38%) | 3.17 (2.84, 3.54) | 6.02 (5.47, 6.63) |
| 24. Renal Dys | Hematologic Severe | 1,277 (0.75%) | 3.17 (2.57, 3.91) | 5.36 (4.44, 6.47) |
| 25. Coagulopathy | Renal Dys | 3,094 (1.81%) | 3.17 (2.74, 3.66) | 6.27 (5.52, 7.12) |
| 26. HTN Complicated | Neuro Other | Wt Loss/Malnut | 1,282 (0.75%) | 3.16 (2.60, 3.85) | 5.98 (4.99, 7.17) |
| 27. Atrial Fib | HTN Complicated | PVD | 4,977 (2.92%) | 3.14 (2.79, 3.54) | 6.30 (5.67, 6.99) |
| 28. HTN Complicated | Neuro Other | PVD | 2,030 (1.19%) | 3.13 (2.64, 3.71) | 5.95 (5.10, 6.95) |
| 29. Neuro Other | Pneumonia | 3,288 (1.93%) | 3.12 (2.71, 3.59) | 5.98 (5.27, 6.78) |
| 30. Heart Failure | Pneumonia | 9,557 (5.60%) | 3.10 (2.82, 3.42) | 6.07 (5.58, 6.61) |
| 31. Neuro Other | PVD | Renal Dys | 2,192 (1.29%) | 3.07 (2.61, 3.61) | 5.99 (5.18, 6.93) |
| 32. COPD | PVD | Renal Dys | 7,797 (4.57%) | 3.07 (2.77, 3.39) | 6.08 (5.56, 6.64) |
| 33. Atrial Fib | Pneumonia | 7,252 (4.25%) | 3.07 (2.76, 3.40) | 6.01 (5.48, 6.59) |
| 34. COPD | HTN Complicated | Neuro Other | 1,981 (1.16%) | 3.05 (2.56, 3.63) | 6.06 (5.18, 7.09) |
| 35. HTN Complicated | Pneumonia | 6,876 (4.03%) | 3.04 (2.73, 3.39) | 6.07 (5.52, 6.68) |
| 36. Coagulopathy | COPD | Wt Loss/Malnut | 977 (0.57%) | 3.03 (2.40, 3.83) | 6.12 (4.98, 7.51) |
| 37. Pneumonia | Renal Dys | 8,287 (4.86%) | 3.03 (2.74, 3.35) | 5.89 (5.40, 6.43) |
| 38. COPD | Neuro Other | PVD | 2,610 (1.53%) | 2.99 (2.56, 3.50) | 5.99 (5.22, 6.88) |
| 39. Blood Dis | HTN Complicated | PVD | 2,286 (1.34%) | 2.99 (2.55, 3.51) | 5.79 (5.02, 6.69) |
| 40. Coagulopathy | Pneumonia | 1,932 (1.13%) | 2.98 (2.49, 3.56) | 5.64 (4.81, 6.61) |
| 41. Blood Dis | COPD | PVD | 3,213 (1.88%) | 2.98 (2.59, 3.43) | 5.88 (5.20, 6.66) |
| 42. Pneumonia | Pulm Circ Disorder | 3,034 (1.78%) | 2.98 (2.57, 3.45) | 5.82 (5.11, 6.63) |
| 43. Heart Failure | Wt Loss/Malnut | 7,574 (4.44%) | 2.95 (2.67, 3.26) | 5.65 (5.18, 6.17) |
| 44. COPD | Hematologic Severe | 1,143 (0.67%) | 2.95 (2.35, 3.70) | 5.35 (4.38, 6.53) |
| 45. Neuro Other | PVD | Wt Loss/Malnutrition | 1,534 (0.90%) | 2.95 (2.45, 3.55) | 5.61 (4.75, 6.62) |
| 46. HTN Complicated | Arr Non-Emerg | Wt Loss/Malnut | 2,967 (1.74%) | 2.94 (2.56, 3.39) | 5.67 (4.99, 6.43) |
| 47. PVD | Pneumonia | 8,626 (5.06%) | 2.94 (2.66, 3.25) | 5.81 (5.33, 6.34) |
| 48. HTN Complicated | Neuro Other | Trauma | 2,013 (1.18%) | 2.94 (2.47, 3.49) | 5.49 (4.68, 6.43) |
| 49. COPD | HTN Complicated | PVD | 6,562 (3.85%) | 2.92 (2.62, 3.26) | 5.85 (5.31, 6.44) |
| 50. Liver Disease | Pneumonia | 3,551 (2.08%) | 2.92 (2.52, 3.39) | 5.38 (4.72, 6.12) |
| 51. Blood Dis | Heart Failure | Trauma | 2,246 (1.32%) | 2.91 (2.47, 3.43) | 5.73 (4.96, 6.62) |
| 52. AMI/Isch Heart | HTN Complicated | Wt Loss/Malnut | 4,377 (2.57%) | 2.90 (2.57, 3.27) | 5.51 (4.94, 6.15) |
| 53. Coagulopathy | COPD | PVD | 1,735 (1.02%) | 2.85 (2.36, 3.44) | 5.79 (4.90, 6.83) |
| 54. Heart Failure | Neuro Other | 4,318 (2.53%) | 2.84 (2.50, 3.24) | 5.57 (4.96, 6.25) |
| 55. HTN Complicated | Neuro Other | Valvular Heart Disease | 1,617 (0.95%) | 2.84 (2.34, 3.44) | 5.29 (4.43, 6.33) |
| 56. Coagulopathy | HTN Complicated | PVD | 1,394 (0.82%) | 2.83 (2.30, 3.47) | 5.93 (4.93, 7.12) |
| 57. Anemia | HTN Complicated | Neuro Other | 2,890 (1.69%) | 2.82 (2.43, 3.28) | 5.37 (4.69, 6.16) |
| 58. COPD | Renal Dys | Valvular Heart Disease | 5,695 (3.34%) | 2.80 (2.49, 3.15) | 5.46 (4.92, 6.05) |
| 59. Atrial Fib | Heart Failure | Trauma | 6,148 (3.60%) | 2.80 (2.50, 3.13) | 5.67 (5.13, 6.26) |
| 60. COPD | HTN Complicated | Valvular Heart Disease | 5,043 (2.96%) | 2.79 (2.46, 3.16) | 5.42 (4.86, 6.05) |
| 61. Atrial Fib | COPD | Pulm Circ Disorder | 2,716 (1.59%) | 2.78 (2.39, 3.25) | 5.75 (5.02, 6.60) |
| 62. Blood Dis | Anemia | Pulm Circ Disorder | 1,165 (0.68%) | 2.77 (2.22, 3.45) | 5.59 (4.60, 6.79) |
| 63. Atrial Fib | Heart Failure | Stroke/TIA | 5,734 (3.36%) | 2.76 (2.46, 3.11) | 5.45 (4.91, 6.04) |
| 64. Atrial Fib | Anemia | HTN Complicated | 7,070 (4.14%) | 2.76 (2.48, 3.07) | 5.45 (4.95, 5.99) |
| 65. Diab Mod/Severe | Neuro Other | PVD | 1,896 (1.11%) | 2.76 (2.28, 3.34) | 5.13 (4.32, 6.09) |
| 66. HTN Complicated | Neuro Other | Stroke/TIA | 2,151 (1.26%) | 2.75 (2.31, 3.28) | 5.14 (4.39, 6.02) |
| 67. Arr Emerg | 5,720 (3.35%) | 2.75 (2.44, 3.10) | 5.30 (4.77, 5.89) |
| 68. AMI/Isch Heart | Atrial Fib | HTN Complicated | 7,598 (4.45%) | 2.74 (2.46, 3.06) | 5.36 (4.88, 5.89) |
| 69. COPD | Renal Dys | Trauma | 6,048 (3.55%) | 2.74 (2.44, 3.07) | 5.47 (4.95, 6.05) |
| 70. Diab Mod/Severe | Pneumonia | 6,104 (3.58%) | 2.72 (2.41, 3.06) | 5.45 (4.91, 6.05) |
| 71. Atrial Fib | Renal Dys | 11,832 (6.94%) | 2.71 (2.47, 2.97) | 5.27 (4.87, 5.72) |
| 72. COPD | HTN Complicated | Trauma | 5,000 (2.93%) | 2.69 (2.38, 3.05) | 5.48 (4.91, 6.11) |
| 73. AMI/Isch Heart | Pneumonia | 11,648 (6.83%) | 2.69 (2.45, 2.96) | 5.20 (4.79, 5.64) |
| 74. Atrial Fib | Diab Mod/Severe | Heart Failure | 5,269 (3.09%) | 2.69 (2.37, 3.05) | 5.29 (4.74, 5.91) |
| 75. COPD | Neuro Other | Valvular Heart Disease | 1,987 (1.16%) | 2.69 (2.24, 3.23) | 5.03 (4.27, 5.94) |
| 76. Pneumonia | Trauma | 8,048 (4.72%) | 2.69 (2.42, 2.99) | 5.28 (4.82, 5.80) |
| 77. Heart Failure | Liver Disease | Valvular Heart Disease | 2,659 (1.56%) | 2.68 (2.27, 3.18) | 5.06 (4.35, 5.89) |
| 78. COPD | Renal Dys | Smoking or Tobacco Abuse | 6,845 (4.01%) | 2.68 (2.40, 3.00) | 5.34 (4.85, 5.88) |
| 79. HTN Complicated | Liver Disease | Arr Non-Emerg | 2,086 (1.22%) | 2.67 (2.21, 3.23) | 5.14 (4.35, 6.08) |
| 80. Atrial Fib | Heart Failure | Anemia | 9,855 (5.78%) | 2.67 (2.42, 2.95) | 5.29 (4.86, 5.76) |
| 81. HTN Complicated | Pulm Circ Disorder | 3,521 (2.06%) | 2.67 (2.31, 3.09) | 5.15 (4.53, 5.87) |
| 82. COPD | Pneumonia | 12,733 (7.46%) | 2.67 (2.43, 2.93) | 5.21 (4.81, 5.64) |
| 83. Heart Failure | Liver Disease | Arr Non-Emerg | 2,579 (1.51%) | 2.66 (2.24, 3.16) | 5.01 (4.30, 5.84) |
| 84. AMI/Isch Heart | COPD | Renal Dys | 10,244 (6.01%) | 2.63 (2.38, 2.90) | 5.17 (4.75, 5.63) |
| 85. AMI/Isch Heart | COPD | HTN Complicated | 8,856 (5.19%) | 2.63 (2.37, 2.91) | 5.16 (4.72, 5.65) |
| 86. Atrial Fib | Diab Mod/Severe | PVD | 4,157 (2.44%) | 2.62 (2.29, 3.00) | 5.29 (4.70, 5.96) |
| 87. Pneumonia | Stroke/TIA | 6,616 (3.88%) | 2.62 (2.34, 2.94) | 5.25 (4.75, 5.80) |
| 88. Arr Non-Emerg | Pneumonia | 7,940 (4.65%) | 2.62 (2.35, 2.91) | 5.08 (4.63, 5.58) |
| 89. Neuro Other | PVD | Valvular Heart Disease | 1,920 (1.13%) | 2.61 (2.18, 3.14) | 4.96 (4.20, 5.85) |
| 90. Diab Mod/Severe | Heart Failure | Trauma | 4,906 (2.88%) | 2.61 (2.29, 2.97) | 5.04 (4.49, 5.66) |
| 91. Atrial Fib | Diab Mod/Severe | Pulm Circ Disorder | 1,527 (0.90%) | 2.60 (2.11, 3.21) | 5.14 (4.27, 6.20) |
| 92. Heart Failure | Renal Dys | 14,568 (8.54%) | 2.60 (2.37, 2.84) | 5.14 (4.76, 5.55) |
| 93. Anemia | Pneumonia | 12,423 (7.28%) | 2.59 (2.37, 2.85) | 5.06 (4.67, 5.48) |
| 94. Heart Failure | HTN Complicated | 13,535 (7.93%) | 2.59 (2.36, 2.84) | 5.14 (4.75, 5.57) |
| 95. HTN Complicated | PVD | Valvular Heart Disease | 5,506 (3.23%) | 2.58 (2.29, 2.92) | 5.06 (4.54, 5.64) |
| 96. Anemia | HTN Complicated | PVD | 9,546 (5.60%) | 2.58 (2.33, 2.85) | 5.04 (4.62, 5.50) |
| 97. Pulm Circ Disorder | Renal Dys | 3,906 (2.29%) | 2.57 (2.24, 2.95) | 5.09 (4.51, 5.75) |
| 98. Pneumonia | Valvular Heart Disease | 7,207 (4.23%) | 2.57 (2.30, 2.87) | 5.06 (4.59, 5.57) |
| 99. Heart Failure | Anemia | Trauma | 8,515 (4.99%) | 2.54 (2.29, 2.82) | 4.93 (4.50, 5.40) |
| 100. COPD | Heart Failure | 16,840 (9.87%) | 2.54 (2.33, 2.77) | 5.03 (4.67, 5.42) |
| 101. HTN Complicated | PVD | Trauma | 5,491 (3.22%) | 2.53 (2.24, 2.86) | 5.02 (4.51, 5.59) |
| 102. PVD | Renal Dys | Trauma | 6,224 (3.65%) | 2.53 (2.26, 2.84) | 5.06 (4.58, 5.59) |
| 103. AMI/Isch Heart | HTN Complicated | PVD | 9,795 (5.74%) | 2.50 (2.26, 2.76) | 4.92 (4.51, 5.38) |
| 104. Heart Failure | PVD | 15,000 (8.79%) | 2.50 (2.28, 2.73) | 4.96 (4.59, 5.35) |
| 105. Heart Failure | Pulm Circ Disorder | 5,602 (3.28%) | 2.49 (2.21, 2.82) | 4.91 (4.41, 5.47) |
| 106. COPD | HTN Complicated | Arr Non-Emerg | 5,332 (3.13%) | 2.49 (2.20, 2.82) | 5.03 (4.51, 5.62) |
| 107. Diab Mod/Severe | HTN Complicated | PVD | 6,092 (3.57%) | 2.48 (2.20, 2.81) | 4.96 (4.45, 5.52) |
| 108. HTN Complicated | Arr Non-Emerg | PVD | 5,681 (3.33%) | 2.45 (2.17, 2.77) | 4.81 (4.32, 5.36) |
| 109. Heart Failure | Stroke/TIA | Trauma | 5,054 (2.96%) | 2.45 (2.15, 2.78) | 4.92 (4.39, 5.50) |
| 110. PVD | Pulm Circ Disorder | 4,079 (2.39%) | 2.42 (2.11, 2.78) | 4.84 (4.28, 5.46) |
| 111. Coagulopathy | Heart Failure | Stroke/TIA | 1,357 (0.80%) | 2.42 (1.94, 3.02) | 4.65 (3.81, 5.67) |
| 112. COPD | Arr Non-Emerg | Pulm Circ Disorder | 2,601 (1.52%) | 2.41 (2.04, 2.85) | 4.93 (4.25, 5.71) |
| 113. Coagulopathy | HTN Complicated | Stroke/TIA | 1,081 (0.63%) | 2.31 (1.80, 2.97) | 4.60 (3.68, 5.76) |
| Any Multimorbid Patient | 61,772 (36.21%) | 1.90 (1.77, 2.04) | 3.72 (3.51, 3.94) |
| From the Excluded Patients | |||
| Any Excluded Patient | 83,615 (32.89%) | 2.05 (1.91, 2.20) | 3.83 (3.60, 4.07) |
| Age ≥ 85 | 41,099 (16.17%) | 3.39 (3.16, 3.63) | 6.84 (6.45, 7.26) |
| Alzheimer’s Disease and Related Disorders | 32,107 (12.63%) | 2.29 (2.11, 2.48) | 4.49 (4.18, 4.82) |
| Metastatic Solid Cancers | 26,754 (10.53%) | 1.88 (1.73, 2.05) | 3.35 (3.11, 3.61) |
A crosswalk between abbreviated and non-abbreviated versions of comorbidity names is available in Supplemental Digital Content Section 1.
Rates for the comorbidity sets reflect the percentage of the study population after exclusions (N = 170,575). Rates for the excluded patients reflect the percentage of the total 2011 general surgical population before exclusions (N = 254,190).
Table 3 provides sample sizes, odds ratios and 95% CIs estimated from the 2011 application dataset, for each of the 113 parsimonious QCSs that were derived in the 2009–2010 dataset. The Mantel-Haenszel odds ratios use two different reference groups. The second-to-last column reports the odds of mortality versus a representative reference group comprising 2011 patients, who had no QCSs or varied QCSs, while the last column uses all non-multimorbid patients as a reference group. Table 3 also provides the group results for all multimorbid patients. As can be seen, multimorbid patients as a group had a 1.90 odds of mortality (95% CI: 1.77, 2.04) versus a general reference population, and a 3.72 odds of mortality (95% CI: 3.51, 3.94) versus the non-multimorbid group. At the bottom, we also report the ORs associated with each excluded population using the same two reference populations as above. For excluded patients, the overall increased odds of mortality versus the general reference group was 2.05 (1.91, 2.20), and the odds of mortality versus non-multimorbid patients was 3.83 (3.60, 4.07). Examining individual excluded subgroups, all displayed significant increases in mortality relative to the reference group, with age ≥ 85 displaying the greatest risk.
It is important to understand that there were many patients who had multiple QCSs, and, as expected, patients with more qualifying comorbidity sets generally had higher mortality rates (see Supplemental Digital Content Section 7). In Supplemental Digital Content 7c, we demonstrate that non-multimorbid patients only began to have comparable risk to multimorbid patients when they had 9 or more of the comorbidities used to define the QCSs.
External matching application using qualifying comorbidity sets
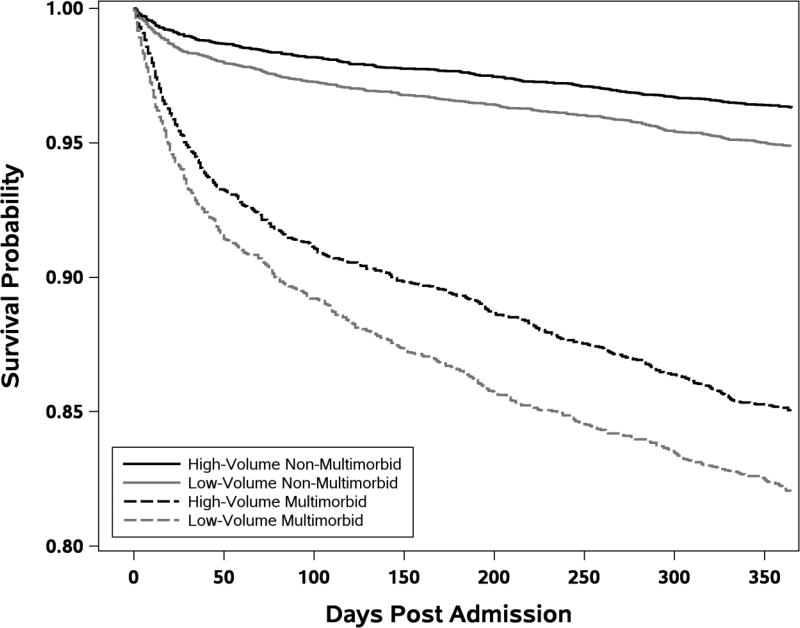
To demonstrate that our newly-developed qualifying comorbidity sets can be applied to a matching study, we matched patients across high-volume hospitals and low-volume hospitals. Patients had higher 30-day mortality at low-volume hospitals, displaying an odds ratio of 1.46 (95% CI 1.21, 1.75), P < 0.0001 versus high-volume hospitals. In 3,151 matched pairs with multimorbidity, the odds of mortality was 1.36 (95% CI 1.09, 1.70), P = 0.006. In 6,387 pairs without multimorbidity, the odds ratio was even higher, 1.67 (95% CI 1.21, 2.30), P = 0.0016. In matched multimorbid patients, the death rate at low-volume hospitals was 6.7% versus 5.2% in high-volume hospitals; in the non-multimorbid group, the death rate was 1.7% in low-volume hospitals versus 1.0% in high-volume hospitals. In Figure 1 we provide Kaplan-Meier survival plots for the multimorbid and non-multimorbid matched pairs at the high-volume and low-volume hospitals. Though we developed our multimorbid definition for 30-day mortality (a common measure of surgical quality), we extend the plot to 1-year mortality to better observe the longer-term follow-up of these vulnerable patients.
FIGURE 1. Kaplan-Meier Plots of Survival after Admission in Multimorbid and Non-Multimorbid Matched General Surgery Patients in Low-Volume and High-Volume Hospitals.
Each matched pair contained one patient from a high-volume hospital and one from a low-volume hospital (N=3,151 MM pairs and N=6,387 non-MM pairs), matched exactly on 135 principal procedures and whether or not the patient was multimorbid, and further matched on risk of mortality on admission, a propensity score for admission to a low-volume hospital, demographics, admission type, 29 comorbidities, and 113 individual qualifying comorbidity sets.
Further Validation Studies
In order to assess the stability of our algorithm for defining multimorbidity, we obtained the 2012 and 2013 surgical patient dataset and re-ran the cross-screening algorithm. The new dataset was similar to our initial 2009 and 2010 dataset, with the exception that starting in October 2010, CMS raised the number of secondary diagnosis fields from 8 to 24. The 2011 dataset served as an out-of-sample dataset for both the original 2009–10 dataset and the replication 2012–13 dataset. In Supplemental Digital Content 8, we provide the 2-by-2 classification table of 2011 patients under each algorithm. Of the 170,575 patients, 159,720 (93.6%) were concordant. When there were discordant pairs, 2,233 (1.3%) were multimorbid under the 2009–10 algorithm, but not under the 2012–13 algorithm, and 8,622 (5.1%) were discordant in the reverse manner. This may be partially explained by the fact that, as mentioned earlier, CMS increased the number of fields available for analysis starting in October 2010.
Comparison of multimorbidity definitions
Finally, we asked if our new definition of multimorbidity provides different information from a well-known existing approach (though one not specifically developed for our eligible general surgery patients). We compared our definitions to those developed by Salive,24 which defined multimorbid patients as having 2 or more comorbidities from a list of 15. Using the 2011 dataset, and applying our same study exclusions, we compared the present study’s definition of multimorbidity to the Salive definition (see Supplemental Digital Content Section 9 for a comparison of the multimorbidity classifications). Among 170,575 patients, the concordance was only 47%. When our definition called a patient multimorbid and the Salive definition did not (N = 1,061), the mortality rate was 4.5%. When our definition suggested no multimorbidity and Salive suggested there was multimorbidity (N = 89,077), the mortality rate was 1.7%.
DISCUSSION
Multimorbidity is a difficult concept to define and, understandably, variations in definitions of multimorbidity are common. We wanted our definitions of qualifying comorbidity sets to be relevant to inpatient general surgical care, so we based our algorithm on the outcome of 30-day all-cause mortality and we excluded patients 85 or older, metastatic solid cancer patients, and patients with dementia to focus our definition on other, less obvious, reasons for poor outcomes. Of interest, these categories of exclusion had comparable risks to our defined multimorbid patients as a whole. Users of our multimorbidity definitions can, if desired, add back these high-risk groups, as such groups had comparable elevated risk to our defined MM group having at least one QCS. We further required our approach to rigorously define what an important elevation of risk would be for a patient defined as having a qualifying comorbidity set. Hence, we wanted a simultaneous 95% confidence interval for the odds ratio associated with any specific qualifying comorbidity set to be above a doubling of the odds of death relative to a defined reference population. We defined comorbidity sets with singles, doubles, and triples, but did not explore quartets or beyond because the number of possibilities would be too large and the data too thin.
The advantage of defining multimorbidity through QCSs, rather than using a risk score to define elevated risk, is that the reasons for being considered high risk can be determined through the QCS designations. While any given risk score can be achieved by a vast number of comorbidity combinations, patients designated as having multimorbidity are defined by their QCSs. Hospitals can determine if outcomes vary by specific QCSs or groups of QCSs, in order to improve quality of care.
Our resulting definition of multimorbidity, the presence of at least one qualifying comorbidity set, performed very well on our 2011 application dataset. We found that as a group, patients categorized as multimorbid had a much higher risk of death than a general reference population or those without multimorbidity. To further study our definition, and to demonstrate that we can perform matching studies leveraging the 113 parsimonious comorbidity sets, we asked whether surgical patients with multimorbidity fared better at high-volume hospitals, since high volume is often associated with better outcomes.43 We found that matching quality was excellent, and we observed significantly elevated mortality at low-volume hospitals. We further replicated our cross-screening algorithm using 2012–2013 patients, and found above 93% concordance with our original definition when we classified out-of-sample 2011 patients. Where there was discordance in defining a patient with multimorbidity, the development dataset that included more secondary diagnoses (2012–13) identified more patients as multimorbid. As datasets and coding systems change over time, users of these algorithms will need to update multimorbidity definitions accordingly.
There are important limitations to our study. The development of multimorbidity definitions is somewhat dependent on the dataset utilized (as demonstrated above), though we did find good stability across the claims data we had access to. In the future, more clinical data may become available and care patterns may change, requiring algorithms to be re-run. Any approach that uses claims data may result in some misclassification of the severity of comorbidities. Our decision to use the more severe state in the comorbidity diabetes and renal failure may have overestimated the severity of these conditions for some patients. Furthermore, there is always the possibility that lack of uniformity in coding may occur, with some hospitals upcoding patients in order to show a greater number of multimorbid patients.
In summary, our study has developed and validated a new definition of multimorbidity based on a methodology that ensures each qualifying comorbidity set has elevated odds of 30-day mortality with high confidence and provides elevated group-wise risk as well. Our hope is that this new approach will aid caregivers treating multimorbid patients and aid policymakers evaluating care for these very complex and vulnerable patients.
Supplementary Material
Table 4. 2011 Application Match Balance Table.
See Supplemental Digital Content 10 for a complete matching table. Note, each matched pair between patients at a low-volume (LV) and a high-volume hospital (HV) was matched exactly on the 135 surgical principal procedures performed at both high-volume and low-volume hospitals, and an indicator of multimorbidity or non-multimorbidity status based on the 113 individual qualifying comorbidity sets.
| Variable (% unless noted) | LV Hosp Cases N = 3,151 |
HV Hosp Matched Controls N = 3,151 |
All HV Hosp Controls N = 20,758 |
Std. Diff‡ Before Match |
P-Value Before Match |
Std. Diff‡ After Match |
P-Value After Match |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age on admission (years, mean) | 75.5 | 75.5 | 75.3 | 0.04 | 0.048 | −0.01 | 0.575 |
| Sex (% Male) | 47.6 | 47.2 | 49.0 | −0.03 | 0.141 | 0.01 | 0.762 |
| Emergency Admission | 46.8 | 46.2 | 39.9 | 0.14 | 0.000 | 0.01 | 0.649 |
| Transfer-in Status | 0.5 | 1.3 | 0.6 | −0.01 | 0.803 | −0.10 | 0.002 |
| Probability of 30-Day Mortality (mean) | 6.4 | 6.5 | 7.8 | −0.12 | 0.000 | −0.01 | 0.634 |
| N QCSs (mean) | 7.7 | 7.5 | 9.0 | −0.12 | 0.000 | 0.02 | 0.197 |
| Selected Qualifying Comorbidity Sets(see Supplemental Digital Content 10 for complete list of 113 matched sets) | |||||||
| COPD | Heart Failure | 28.7 | 29.0 | 26.1 | 0.06 | 0.002 | −0.01 | 0.781 |
| Heart Failure | PVD | 22.2 | 23.7 | 24.3 | −0.05 | 0.010 | −0.04 | 0.168 |
| Heart Failure | Renal Dys | 21.9 | 22.3 | 23.5 | −0.04 | 0.047 | −0.01 | 0.693 |
| Heart Failure | HTN Comp | 19.8 | 19.5 | 21.8 | −0.05 | 0.009 | 0.01 | 0.824 |
| COPD | Pneumonia | 22.9 | 22.1 | 19.8 | 0.07 | 0.000 | 0.02 | 0.469 |
| Anemia | Pneumonia | 20.0 | 19.5 | 20.3 | −0.01 | 0.704 | 0.01 | 0.591 |
| Atrial Fib | Renal Dys | 16.0 | 16.9 | 19.8 | −0.10 | 0.000 | −0.02 | 0.359 |
| AMI/Isch Heart | Pneumonia | 19.3 | 18.9 | 18.9 | 0.01 | 0.575 | 0.01 | 0.677 |
| AMI/Isch Heart | COPD | Renal Dys | 15.2 | 15.8 | 16.2 | −0.03 | 0.145 | −0.02 | 0.486 |
| Atrial Fib | Heart Failure | Anemia | 13.1 | 14.7 | 16.8 | −0.10 | 0.000 | −0.05 | 0.069 |
| AMI/Isch Heart | HTN Comp | PVD | 13.3 | 14.5 | 16.3 | −0.08 | 0.000 | −0.03 | 0.178 |
| Heart Failure | Pneumonia | 15.9 | 14.4 | 15.2 | 0.02 | 0.276 | 0.04 | 0.106 |
| Anemia | HTN Comp | PVD | 11.5 | 13.2 | 15.9 | −0.13 | 0.000 | −0.05 | 0.047 |
| AMI/Isch Heart | COPD | HTN Comp | 13.2 | 13.7 | 14.1 | −0.03 | 0.177 | −0.02 | 0.530 |
| PVD | Pneumonia | 13.0 | 12.7 | 13.9 | −0.03 | 0.202 | 0.01 | 0.707 |
| Heart Failure | Anemia | Trauma | 12.4 | 12.3 | 14.1 | −0.05 | 0.013 | 0.00 | 0.909 |
| Pneumonia | Renal Dys | 12.1 | 10.6 | 13.4 | −0.04 | 0.045 | 0.04 | 0.074 |
| Pneumonia | Trauma | 14.0 | 12.8 | 12.9 | 0.03 | 0.078 | 0.04 | 0.172 |
| Arr Non-Emerg | Pneumonia | 11.9 | 11.6 | 13.3 | −0.04 | 0.041 | 0.01 | 0.667 |
| COPD | PVD | Renal Dys | 11.6 | 11.9 | 12.3 | −0.02 | 0.293 | −0.01 | 0.696 |
| AMI/Isch Heart | Atrial Fib | HTN Comp | 8.8 | 9.6 | 12.9 | −0.13 | 0.000 | −0.03 | 0.257 |
| Heart Failure | Wt Loss/Malnut | 11.7 | 10.8 | 12.7 | −0.03 | 0.089 | 0.03 | 0.319 |
| Atrial Fib | Pneumonia | 10.8 | 10.6 | 11.9 | −0.04 | 0.075 | 0.01 | 0.839 |
| Pneumonia | Valvular Heart Disease | 9.6 | 9.7 | 12.2 | −0.08 | 0.000 | 0.00 | 0.932 |
| Atrial Fib | Anemia | HTN Comp | 7.7 | 8.7 | 12.1 | −0.15 | 0.000 | −0.03 | 0.168 |
| HTN Comp | Pneumonia | 9.3 | 8.0 | 11.0 | −0.06 | 0.005 | 0.04 | 0.073 |
| COPD | Renal Dys | Smoking/Tobacco Abuse | 10.1 | 10.5 | 10.7 | −0.02 | 0.291 | −0.01 | 0.590 |
| Pneumonia | Stroke/TIA | 11.2 | 11.5 | 10.4 | 0.03 | 0.169 | −0.01 | 0.691 |
| COPD | HTN Comp | PVD | 9.8 | 9.8 | 10.1 | −0.01 | 0.568 | 0.00 | 1.000 |
| Atrial Fib | COPD | PVD | 9.2 | 10.2 | 10.5 | −0.04 | 0.024 | −0.04 | 0.173 |
| PVD | Renal Dys | Trauma | 8.1 | 8.5 | 10.2 | −0.07 | 0.000 | −0.01 | 0.615 |
| Atrial Fib | Heart Failure | Trauma | 8.7 | 9.4 | 10.2 | −0.05 | 0.011 | −0.02 | 0.356 |
| Diab Mod/Severe | Pneumonia | 8.8 | 8.6 | 9.9 | −0.04 | 0.071 | 0.01 | 0.823 |
| Diab Mod/Severe | HTN Comp | PVD | 8.3 | 9.3 | 9.7 | −0.05 | 0.010 | −0.04 | 0.142 |
| COPD | Renal Dys | Trauma | 9.0 | 7.8 | 9.5 | −0.02 | 0.413 | 0.04 | 0.103 |
Standardized Difference in units of standard deviations.
Acknowledgments
FUNDING SOURCE: NIAR56-AG048132
Footnotes
CONFLICT OF INTEREST: None
Supplemental Digital Content 1.docx
References
- 1.American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: An approach for clinicians. J Am Geriatr Soc. 2012;60:E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 3.Center for Medicare & Medicaid Services. Chronic Conditions among Medicare Beneficiaries Chartbook, 2012 Edition. Baltimore, MD: 2012. [Accessed August 9, 2017]. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Downloads/2012Chartbook.pdf. [Google Scholar]
- 4.Marengoni A, Rizzuto D, Wang HX, et al. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 5.Starfield B, Kinder K. Multimorbidity and its measurement. Health Policy. 2011;103:3–8. doi: 10.1016/j.healthpol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Tooth L, Hockey R, Byles J, et al. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol. 2008;61:151–159. doi: 10.1016/j.jclinepi.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Librero J, Peiro S, Ordinana R. Chronic comorbidity and outcomes of hospital care: Length of stay, mortality, and readmission at 30 and 365 days. J Clin Epidemiol. 1999;52:171–179. doi: 10.1016/s0895-4356(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 8.Zekry D, Loures Valle BH, Graf C, et al. Prospective comparison of 6 comorbidity indices as predictors of 1-year post-hospital discharge institutionalization, readmission, and mortality in elderly individuals. J Am Med Dir Assoc. 2012;13:272–278. doi: 10.1016/j.jamda.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Byles JE, D'Este C, Parkinson L, et al. Single index of multimorbidity did not predict multiple outcomes. J Clin Epidemiol. 2005;58:997–1005. doi: 10.1016/j.jclinepi.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(Suppl 3):403–407. doi: 10.1007/s11606-007-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 12.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496–2504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 13.Condelius A, Edberg AK, Jakobsson U, et al. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch Gerontol Geriatr. 2008;46:41–55. doi: 10.1016/j.archger.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Chi MJ, Lee CY, Wu SC. Multiple morbidity combinations impact on medical expenditures among older adults. Arch Gerontol Geriatr. 2011;52:e210–214. doi: 10.1016/j.archger.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Landi F, Liperoti R, Russo A, et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol. 2010;63:752–759. doi: 10.1016/j.jclinepi.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Akner G. Analysis of multimorbidity in individual elderly nursing home residents. Development of a multimorbidity matrix. Arch Gerontol Geriatr. 2009;49:413–419. doi: 10.1016/j.archger.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: A retrospective cohort study. Br J Gen Pract. 2011;61:e12–21. doi: 10.3399/bjgp11X548929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schram MT, Frijters D, van de Lisdonk EH, et al. Setting and registry characteristics affect the prevalence and nature of multimorbidity in the elderly. J Clin Epidemiol. 2008;61:1104–1112. doi: 10.1016/j.jclinepi.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 20.Boyd CM, Fortin M. Future of multimorbidity research: How should understanding of multimorbidity inform health system design? Public Health Reviews. 2010;32:451–474. [Google Scholar]
- 21.Norris SL, High K, Gill TM, et al. Health care for older Americans with multiple chronic conditions: A research agenda. J Am Geriatr Soc. 2008;56:149–159. doi: 10.1111/j.1532-5415.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 22.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichol MB, Knight TK, Priest JL, et al. Nonadherence to clinical practice guidelines and medications for multiple chronic conditions in a California Medicaid population. J Am Pharm Assoc (2003) 2010;50:496–507. doi: 10.1331/JAPhA.2010.09123. [DOI] [PubMed] [Google Scholar]
- 24.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 25.Werner RM, Greenfield S, Fung C, et al. Measuring quality of care in patients with multiple clinical conditions: Summary of a conference conducted by the Society of General Internal Medicine. J Gen Intern Med. 2007;22:1206–1211. doi: 10.1007/s11606-007-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45:480–488. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 27.Glynn LG, Valderas JM, Healy P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract. 2011;28:516–523. doi: 10.1093/fampra/cmr013. [DOI] [PubMed] [Google Scholar]
- 28.Parekh AK, Goodman RA, Gordon C, et al. Managing multiple chronic conditions: A strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126:460–471. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 30.Wong A, Boshuizen HC, Schellevis FG, et al. Longitudinal administrative data can be used to examine multimorbidity, provided false discoveries are controlled for. J Clin Epidemiol. 2011;64:1109–1117. doi: 10.1016/j.jclinepi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Fortin M, Hudon C, Haggerty J, et al. Prevalence estimates of multimorbidity: A comparative study of two sources. BMC Health Serv Res. 2010;10:111. doi: 10.1186/1472-6963-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd CM, Reider L, Frey K, et al. The effects of guided care on the perceived quality of health care for multi-morbid older persons: 18-month outcomes from a cluster-randomized controlled trial. J Gen Intern Med. 2010;25:235–242. doi: 10.1007/s11606-009-1192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross CP, Guo Z, McAvay GJ, et al. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006;54:1898–1904. doi: 10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 34.de Bruin SR, Versnel N, Lemmens LC, et al. Comprehensive care programs for patients with multiple chronic conditions: A systematic literature review. Health Policy. 2012;107:108–145. doi: 10.1016/j.healthpol.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Boult C, Reider L, Frey K, et al. Early effects of "Guided Care" on the quality of health care for multimorbid older persons: a cluster-randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2008;63:321–327. doi: 10.1093/gerona/63.3.321. [DOI] [PubMed] [Google Scholar]
- 36.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 37.Sorace J, Wong HH, Worrall C, et al. The complexity of disease combinations in the Medicare population. Popul Health Manag. 2011;14:161–166. doi: 10.1089/pop.2010.0044. [DOI] [PubMed] [Google Scholar]
- 38.Noyes K, Liu H, Temkin-Greener H. Medicare capitation model, functional status, and multiple comorbidities: model accuracy. Am J Manag Care. 2008;14:679–690. [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman B, Jiang HJ, Elixhauser A, et al. Hospital inpatient costs for adults with multiple chronic conditions. Med Care Res Rev. 2006;63:327–346. doi: 10.1177/1077558706287042. [DOI] [PubMed] [Google Scholar]
- 40.Ajmera M, Wilkins TL, Findley PA, et al. Multimorbidity, mental illness, and quality of care: Preventable hospitalizations among Medicare beneficiaries. Int J Family Med. 2012;2012:823294. doi: 10.1155/2012/823294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7:82. doi: 10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starfield B, Lemke KW, Herbert R, et al. Comorbidity and the use of primary care and specialist care in the elderly. Ann Fam Med. 2005;3:215–222. doi: 10.1370/afm.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Q, Small DS, Rosenbaum PR. Cross-screening in observational studies that test many hypotheses. J Am Stat Assoc. in press. [Google Scholar]
- 45.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 46.SAS Institute Inc. Version 9.4 of the Statistical Analytic Software System for UNIX. Cary, NC: 2013. [Google Scholar]
- 47.Pimentel SD, Kelz RR, Silber JH, et al. Large, sparse optimal matching with refined covariate balance in an observational study of the health outcomes produced by new surgeons. J Am Stat Assoc. 2015;110:515–527. doi: 10.1080/01621459.2014.997879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 49.Pimentel SD. Large sparse optimal matching with R package rebalance. Obs Studies. 2016;2:4–23. [Google Scholar]
- 50.Bishop YMM, Fienberg SE, Holland PW. Discrete Multivariate Analysis: Theory and Practice. Cambridge, MA: The MIT Press; 1975. Chapter 8: Analysis of square tables: Symmetry and marginal homogeneity; pp. 281–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.