Abstract
Infantile systemic hyalinosis (ISH) is a rare autosomal recessive disease. Typically, ISH patients present with progressive painful joint contractures, intractable diarrhea, hyperpigmented skin lesions, and perianal fleshy nodules. We report a case of a 19-month-old male child with atypical ISH presentation. His main clinical finding was protein-losing enteropathy due to intestinal lymphangectasia. This report is intended to enhance awareness about the gastrointestinal tract presentation of ISH.
Infantile systemic hyalinosis (ISH) is a rare progressive autosomal recessive disease characterized by a widespread hyaline material deposition in skin, the musculoskeletal system, and internal organs. ISH usually appears in early infancy with progressive painful joint contractures, failure to thrive, hyperpigmentation over the bony prominences, gingival hyperplasia, and perianal fleshy nodules. It is a fatal disease and most patients die before the second birthday.1–3 Gastrointestinal symptoms, particularly intractable diarrhea, are important features of the disease, and are the result of deposition of a hyaline material in the intestinal wall. Typically patients fail to thrive because of malabsorption. However, rarely patients may present with protein-losing enteropathy due to intestinal lymphangectasia.4 We describe a case of a male child with atypical ISH, proved by molecular genetic testing, who presented with protein-losing enteropathy that was secondary to intestinal lymphangectasia. This report is intended to enhance awareness about the gastrointestinal tract presentation of ISH.
CASE
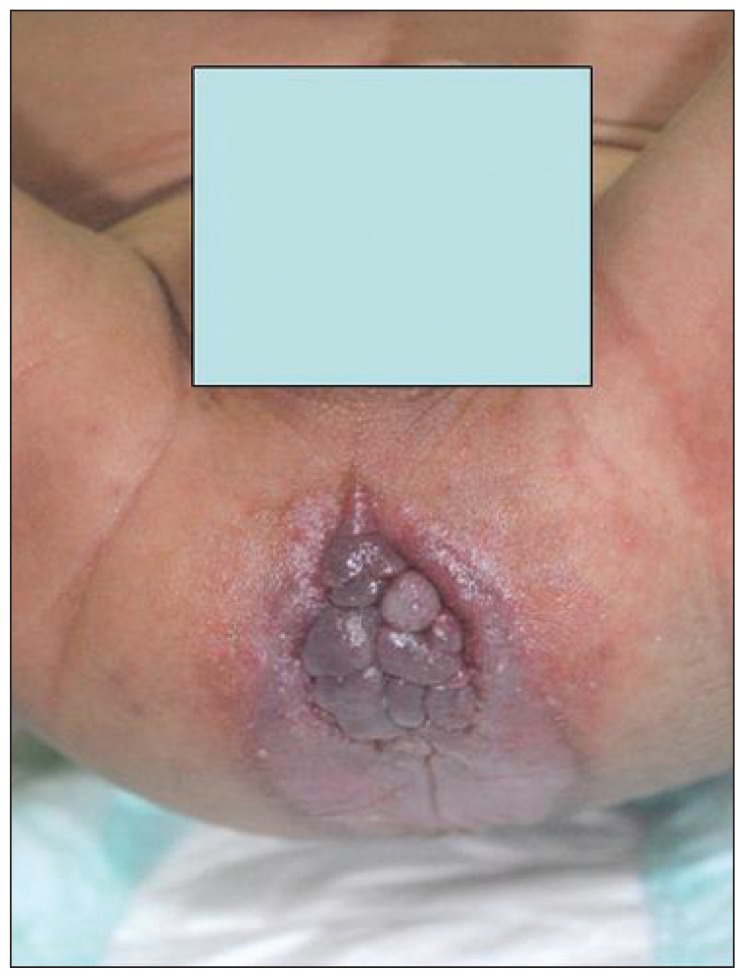
Our patient was a 19-month-old male child. He was the sixth child of consanguineous parents, with a positive family history of two cousins having died in early infancy owing to generalized stiffness and diarrhea with no definite diagnosis. He was born at 31 weeks gestational age by cesarean delivery because of fetal distress. He was admitted to the neonatal intensive care unit, and required mechanical ventilation because of respiratory distress. After a couple of weeks, he was discharged in a stable general condition. However, at the age of 6 months, he developed intractable diarrhea 6 to 8 times per day, that was watery, of large volume, greasy, and foul smelling. His parents noticed that the child would cry and was irritable during handling, and they also observed perianal fleshy nodules. However, his joints were not stiff. Delayed speech was observed with normal hearing, vision, and cognitive function. Physical examination revealed dysmorphic features in the form of a triangular face, synophrys, prominent ears, gingival hypertrophy, and a high-arched palate. His growth parameters were below the 5th percentile. He had hyperpigmentation over the metacarpophalangeal joints and multiple subcutaneous nodules on the chest and abdomen in addition to perianal fleshy nodules (Figure 1).
Figure 1.
Perianal fleshy nodules.
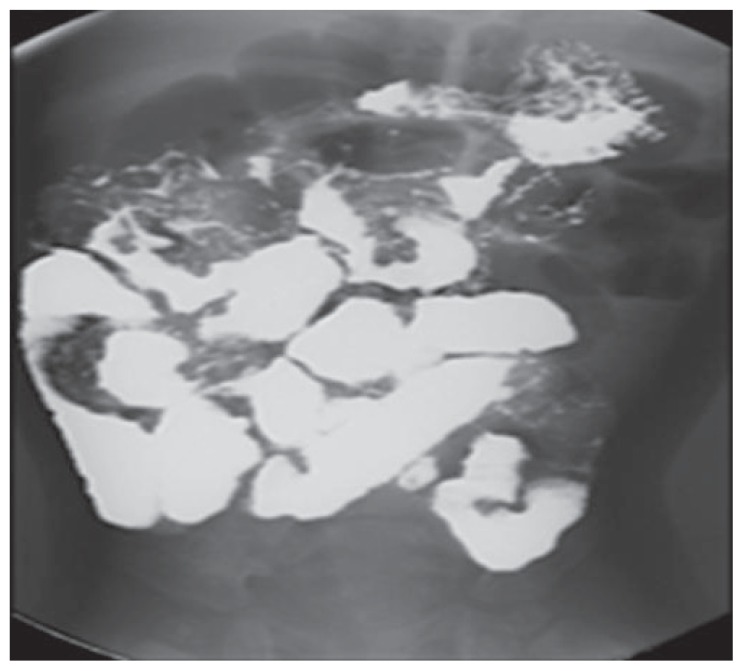
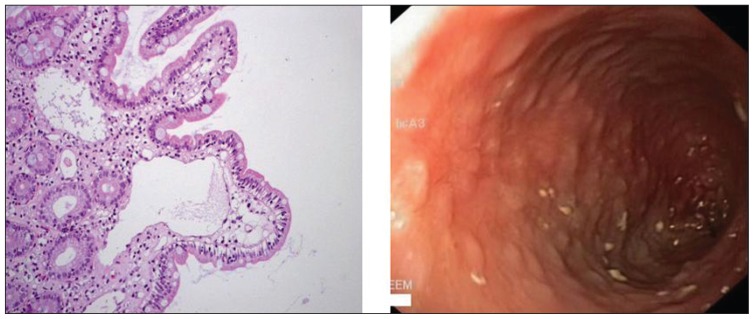
Laboratory results showed a white blood cell count of 17.5×109/L (normal, 5–15×109/L), hemoglobin 93 g/L (normal, 120–200 g/L), and a platelet count of 830×109/L (normal, 200–550×109/L). The erythrocyte sedimentation rate was 25 mm/h (normal, 0–5 mm/h). Liver function tests were unremarkable. However; the serum albumin was 19 g/L (normal, 37–49 g/L). The stool was negative for ova and parasite, Cryptosporidium, Clostridium difficile toxin, and cultures. Tissue transglutaminase and endomysial antibodies were normal. A skeletal survey showed diffuse osteopenia with symmetrical bilateral subperiosteal bone loss. A barium meal and small-bowel follow through study showed a thickened stomach wall and small bowel loops with a polypoid-like appearance projecting in the lumen of the small bowel loops, which raised the suspicion of lymphangectasia (Figure 2). Upper gastrointestinal endoscopy showed nodularity with whitish spots in the duodenum. Histopathology from these lesions showed dilated lymphatic vessels in the mucosa, confirming the diagnosis of intestinal lymphangectasia (Figure 3). Gene studies showed a homozygous missense mutation in the anthrax toxin receptor-2 (ANTXR2) gene.
Figure 2.
Barium meal and small bowel follow-through showing thickened wall of the stomach and small-bowel loops with polypoid-like appearance projecting in the lumen of the small-bowel loops.
Figure 3.
Upper gastrointestinal endoscopy showing nodularity with whitish spots in the duodenum. Histopathology from these lesions showed dilated lymphatic vessels in the mucosa, confirming the diagnosis of intestinal lymphangectasia.
The patient did not improve on dietary modification using the medium-chain triglycerides (MCTs) formula. Accordingly, he was started on cyclic total parenteral nutrition, but unfortunately he passed away at home because of severe infection.
DISCUSSION
ISH is a rare autosomal recessive connective tissue disorder characterized by widespread deposition of hyaline material in many tissues, including the musculoskeletal system and gastrointestinal tract.5 The presentation is usually within the first few weeks of life with progressive painful, swollen joint contractures and mucocutaneous lesions in the form of hyperpigmentation over the bony prominences, and subcutaneous nodules, gingival and perianal fleshy tumor-like lesions.3
Intractable diarrhea is a common presenting manifestation of ISH. Patients usually present with intractable diarrhea, edema, and hypoalbuminemia. Protein-losing enteropathy has been described in many cases of ISH. Most of the reported cases are similar with gastrointestinal tract symptoms such as chronic diarrhea and malabsorption due to the deposition of hyaline material in the intestinal wall. However, intestinal lymphangiectasia can be the underlying cause of protein-losing enteropathy in ISH.5–7 Our patient presented with clinical features suggestive of protein-losing enteropathy, which was supported by a barium meal and follow-through study and confirmed by histopathology. The diagnosis of ISH was supported by the mucocutaneous and gastrointestinal tract features, but not by the musculoskeletal involvement, which was absent in this case. He did not have the usual progressive painful joint contractures. However, molecular genetic testing revealed a homozygous missense mutation in the ANTXR2 gene.8
99mTC-human serum albumin (99mTC-HSA) scan is useful in the evaluation of children with proteinlosing enteropathy. Negative results from the scan are not unexpected, as the disease could be focal or mild as reflected by biochemical findings. Two patients from our ISH cohort underwent this procedure. The 99mTC-HSA scan was negative. Accordingly we did not request the 99mTC-HSA scan for the current patient; however, he underwent upper gastrointestinal endoscopy, and the diagnosis of intestinal lymphangiectasia was established by the characteristic histology of grossly dilated lymphatics seen in the lamina propria of the small bowel.6,9 To the best of our knowledge, no similar cases of intestinal lymphangiectasia in ISH have been documented in published reports.
Intestinal lymphangiectasia is an important cause of protein-losing enteropathy. It can be either idiopathic or is often seen in association with other disease entities. Unfortunately, no cure is available for it. Lifelong dietary modifications with high protein, restricted fat substituted with MCT, and vitamin supplements remain the mainstay of management of intestinal lymphangiectasia. 10,11 The prognosis of the ISH patient is very poor, and the presence of intestinal lymphangiectasia complicates the outcome.
REFERENCES
- 1.Glover M, Lake B, Atherton D. Infantile systemic hyalinosis: newly recognized disorder of collagen? Pediatrics. 1991;87:228–234. [PubMed] [Google Scholar]
- 2.Stucki U, Spycher MA, Eich G, Rossi A, Sacher P, Steinmann B, et al. Infantile systemic hyalinosis in siblings: Clinical report, biochemical and ultrastructural findings, and review of the literature. Am J Med Genet. 2001;100:122–129. doi: 10.1002/1096-8628(20010422)100:2<122::aid-ajmg1236>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mayouf SM, AlMehaidib A, Bahabri S, Shabib S, Sakati N, Teebi AS. Infantile systemic hyalinosis: A fatal disorder commonly diagnosed among Arabs. Clin Exp Rheumatol. 2005;23:717–720. [PubMed] [Google Scholar]
- 4.Büyükgebiz B, Oztürk Y, Arslan N, Ozer E. A rare cause of protein-losing enteropathy and growth retardation in infancy: Infantile systemic hyalinosis. Turk J Pediatr. 2003;45:258–260. [PubMed] [Google Scholar]
- 5.Landing B, Nadorra R. Infantile systemic hyalinosis: Report of four cases of disease, fatal in infancy, apparently different from juvenile systemic hyalinosis. Pediat Pathol. 1986;6:55–79. doi: 10.3109/15513818609025925. [DOI] [PubMed] [Google Scholar]
- 6.Shields E, Tucker T, Meyers W, Chung CJ. Visualization of protein losing enteropathy in infantile systemic hyalinosis with Tc-99m HSA after albumin challenge. Clin Nucl Med. 1996;21:415–416. doi: 10.1097/00003072-199605000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Aghighi Y, Bahremand S, Nematollahi L. Infantile systemic hyalinosis: Report of three Iranian children and review of the literature. Clin Rheumatol. 2007;26:128–130. doi: 10.1007/s10067-005-0124-y. [DOI] [PubMed] [Google Scholar]
- 8.Deuquet J, Abrami L, Difeo A, Ramirez M, Martignetti J, van der Goot F. Systemic hyalinosis mutations in the CMG2 ectodomain leading to loss of function through retention in the endoplasmic reticulum. Hum Mutat. 2009;30:583–589. doi: 10.1002/humu.20872. [DOI] [PubMed] [Google Scholar]
- 9.Halaby H, Bakheet S, Shabib S, Powe J, Al Mehaidib A, Nazer H. 99mTc-human serum albumin scans in children with protein-losing enteropathy. J Nucl Med. 2000;41:215–219. [PubMed] [Google Scholar]
- 10.Suresh N, Ganesh R, Sankar J, Sathiyasekaran M. Primary intestinal lymphangiectasia. Indian Pediatr. 2009;46:903–906. [PubMed] [Google Scholar]
- 11.Pratz K, Dingli D, Smyrk T, Lust JA. Intestinal lymphangiectasia with protein-losing enteropathy in Waldenstrom macroglobulinemia. Medicine (Baltimore) 2007;86:210–214. doi: 10.1097/MD.0b013e31812e5242. [DOI] [PubMed] [Google Scholar]