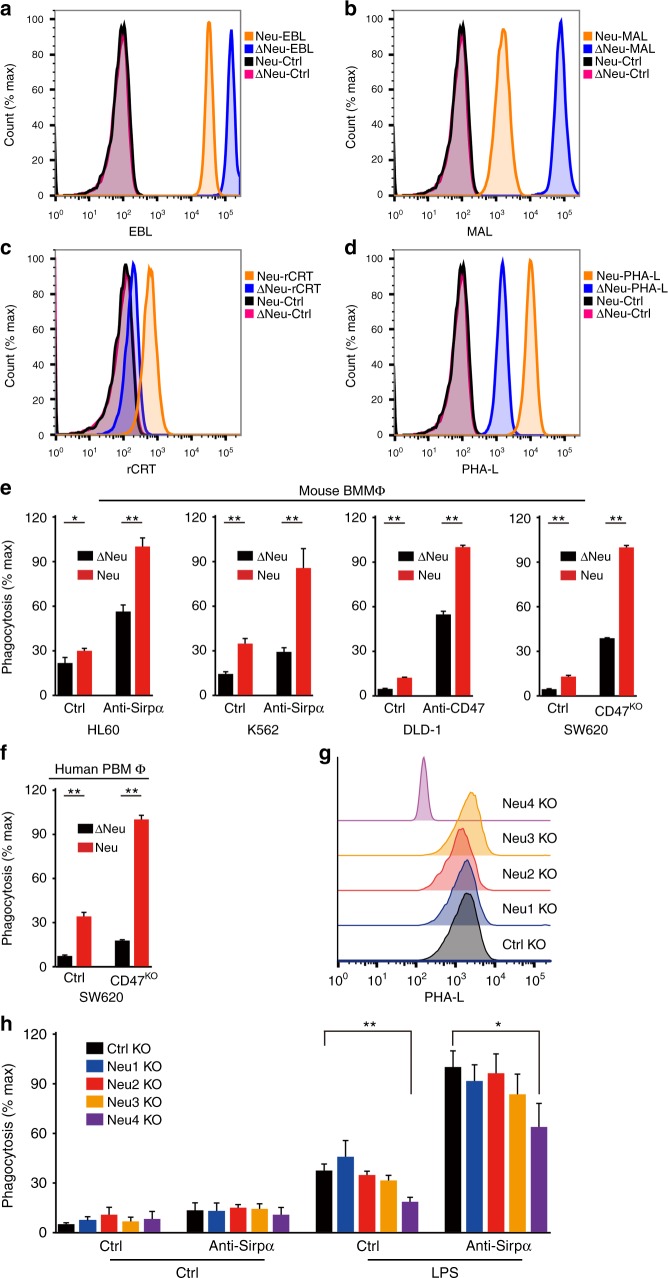
Fig. 5.
Cell surface asialoglycans regulates CRT-mediated PrCR. a, b Treatment with neuraminidase led to the removal of sialic acids from the cell surface of HL60 cells. HL60 cells were treated with heat inactivated neuraminidase (Δneu) or neuraminidase (neu). Cell surface sialic acids were examined by staining with EBL (a) and MAL (b) by flow cytometry analysis. EBL, Elderberry Bark Lectin; MAL, Maackia Amurensis Lectin II. c, d Examination of cell surface CRT and PHA-L binding sites on cancer cells. HL60 cells were treated with heat inactivated neuraminidase (Δneu) or neuraminidase (neu). Recombinant CRT (c) and PHA-L (d) binding after treatment were measured by flow cytometry. e, f Phagocytosis of cancer cells with neuraminidase treatment. In vitro Phagocytosis assays were performed with HL60, K562, DLD-1, and SW620 cells treated with heat inactivated neuraminidase (Δneu) or neuraminidase (neu) as target cells. Mouse bone marrow-derived (e) and human peripheral blood monocyte-derived (f) macrophages were used for the assay. Phagocytosis was normalized to the maximal response in the experiments. n = 3. *P < 0.05, **P < 0.01 (t-test) for phagocytosis between heat inactivated neuraminidase (Δneu)- and neuraminidase (neu)-treated groups. Error bars represent standard deviation. g, h Effects of suppressing the expression of endogenous neuraminidases in cancer cells. Neu1–Neu4 gene knockout were performed with CRISPR in HL60 cells. In vitro Phagocytosis assays were performed with HL60 cells as target cells and mouse bone marrow-derived macrophages (h). Macrophages were treated with PBS (ctrl) or lipopolysaccharide (LPS). Neu4 knockout led to the decrease of cell surface CRT-binding sites and inhibited cancer cell phagocytosis. Phagocytosis was normalized to the maximal response in the experiments. n = 3. *P < 0.05, **P < 0.01 (t-test) for phagocytosis between ctrl and Neu4 KO groups. Error bars represent standard deviation