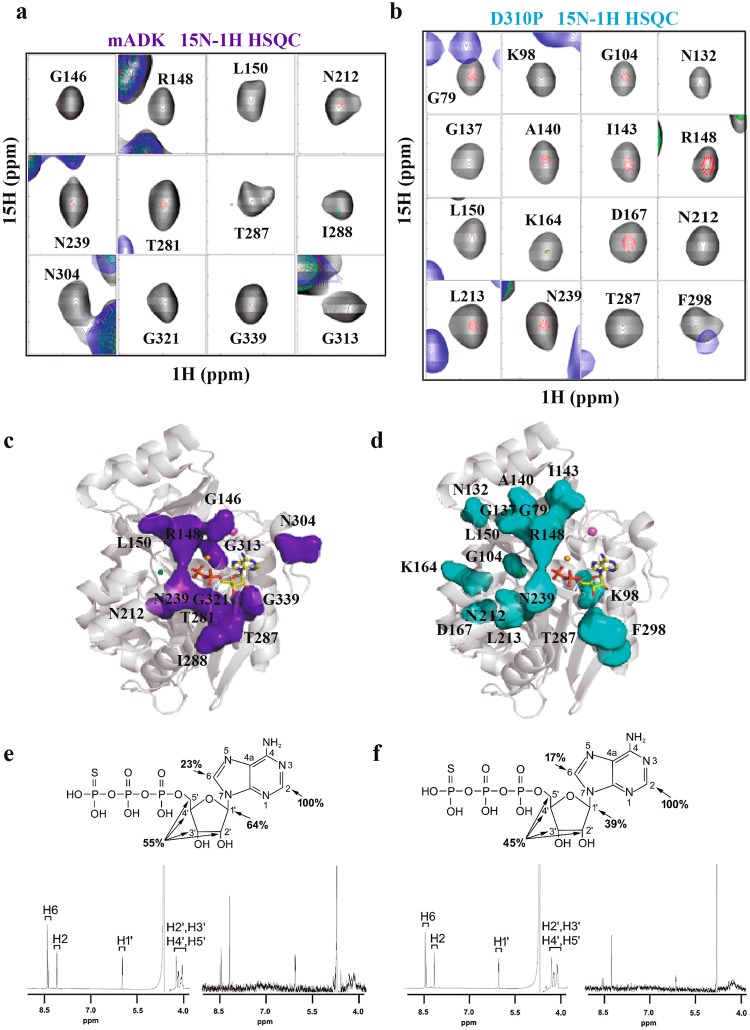
Figure 6.
D310P mutation disrupts K+ sensitivity of mADK. (a) mADK and (b) D310P mutant analyzed by 2D 15N-1H HSQC. In the 2D 15N-1H HSQC spectra, the black, red, green and blue peaks represent chemical shifts changed ATPγS titration ratio of: free WT in black; 1:0.05 in red; 1:0.1 in green and 1:0.2 in blue. For the free D310P mutant, peak is black, followed by 1:0.1 in red, 1:0.2 in green and 1:0.5 in blue. (c) and (d) represent mapping of the broadened residues for mADK and D310P mutant respectively. Broadened residues are featured using as a model, the ternary mADK complex structure (PDB 5KB5). 1D 1H STD NMR spectra of ATPγS interaction with mADK (e) and D310P (f). Enhancements are referred to as 100% to the H-2 resonance of ATPγS. Upper part shows the ATPγS structure with the number for each proton and the % STD enhancement.