Fig 1. Type I IFN-dependent pathway and ER stress response-induced pathway of IFNAR1 degradation.
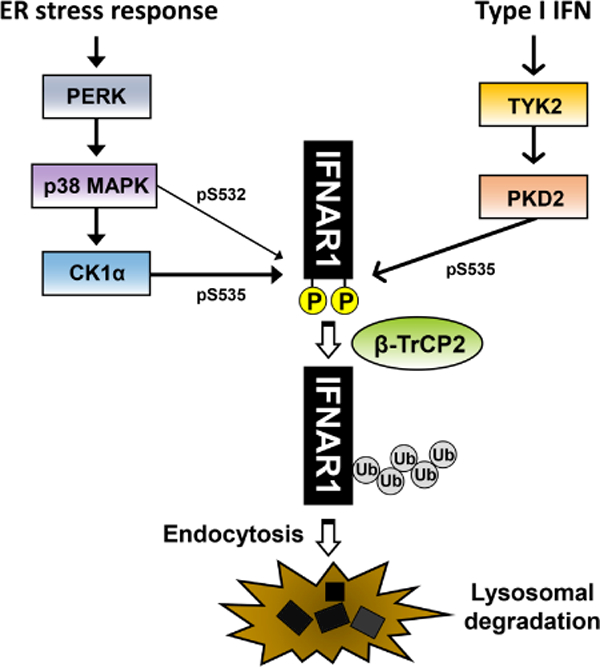
Two representative pathways of IFNAR1 degradation, i.e., ligand (IFN)-dependent pathway and ligand-independent but ER stress-induced pathway are depicted. PERK-mediated ER stress response activates p38 MAPK. IFNAR1 is phosphorylated by p38 MAPK at S532, and then subsequently phosphorylated again by CK1α at S535. Binding of type I IFN to the receptor also induces phosphorylation of IFNAR1 at S535, which is catalyzed by PKD2 and requires the activity of TYK2. The phosphorylation of IFNAR1 then recruits the E3 ubiquitin ligase β-TrCP2, which catalyzes poly-ubiquitination on IFNAR1, leading to the endocytosis and lysosomal degradation of IFNAR1.
