Fig 2. Influenza HA-induced degradation of IFNAR1 blocks the IFNAR signaling pathway.
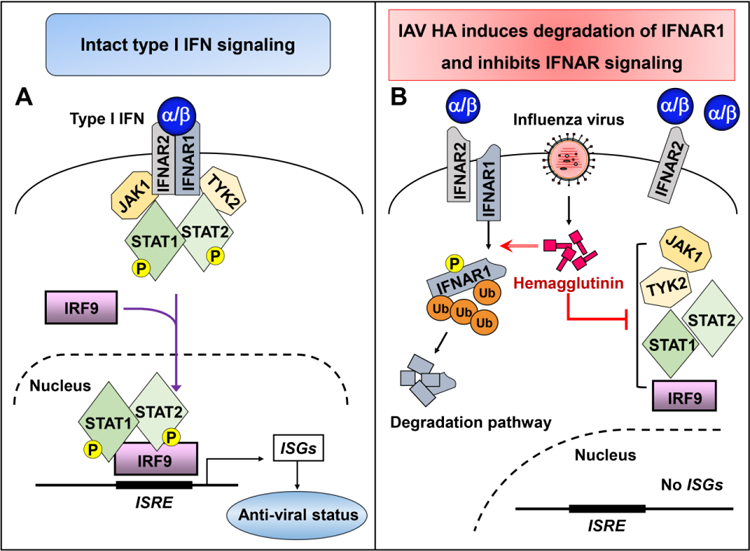
(A)Type I IFNs (IFN-α/β) bind to their receptor IFNAR, which is composed of IFNAR1 and IFNAR2. This interaction elicits the JAK/STAT signaling pathway. JAK1 and TYK2 phosphorylate STAT1 and STAT2, which form a trimeric complex with IRF9, translocate to the nucleus, and bind to interferon stimulated response elements (ISRE), leading to the expression of interferon-stimulated genes (ISGs). ISGs encode antiviral proteins, which establish an anti-viral status. (B) During IAV infection, hemagglutinin protein induces phosphorylation and ubiquitination of IFNAR1, leading to the degradation of this receptor. This process will deplete cellular IFNAR1, which in turn suppresses the JAK/STAT activation and inhibits the expression of ISGs.
