Figure 1.
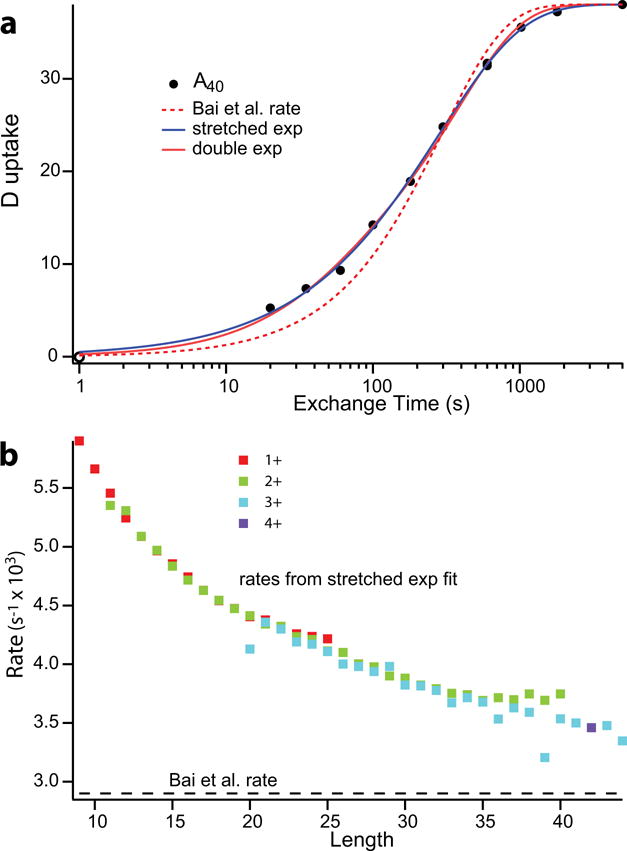
PDLA exchange: (a) Deuterium uptake of a 40 residue peptide from random heterogeneously polymerized D,L-alanine. PDLA samples were exposed to H to D exchange (15°C in 90% D2O at pDread of 4.01) for increasing times, samples quenched at pH 2.4, 0°C to essentially halt exchange, and injected into an online sytem where they were desalted on a small C8 column, and separated by size in a C18 column. The eluant was introduced by electrospray into a Thermo LTQ Orbitrap XL mass spectrometer which further separates peptides by mass and allows time-dependent deuterium uptake of the differently sized PDLA peptides to be measured. Deuterium loss due to back exchange during the analysis was corrected for using an “all D” sample initially fully exchanged in the same buffer. The point shown at 1 sec is a zero time control which was injected immediately after simultaneous addition of quench and D2O buffer. The dashed line shows the single exponential rate predicted from the Bai et al. parameters for large poly peptides. The blue and red solid lines show single stretched exponential and double exponential fits. (b) Stretched exponential rates as a function of PDLA polymer length. The dashed line along the bottom indicates the reference rate from Bai et al. (9) for large poly peptides.
