Fig. 2. Expression of laforin mutated forms in primary fibroblasts from the Y112X/N163D patient.
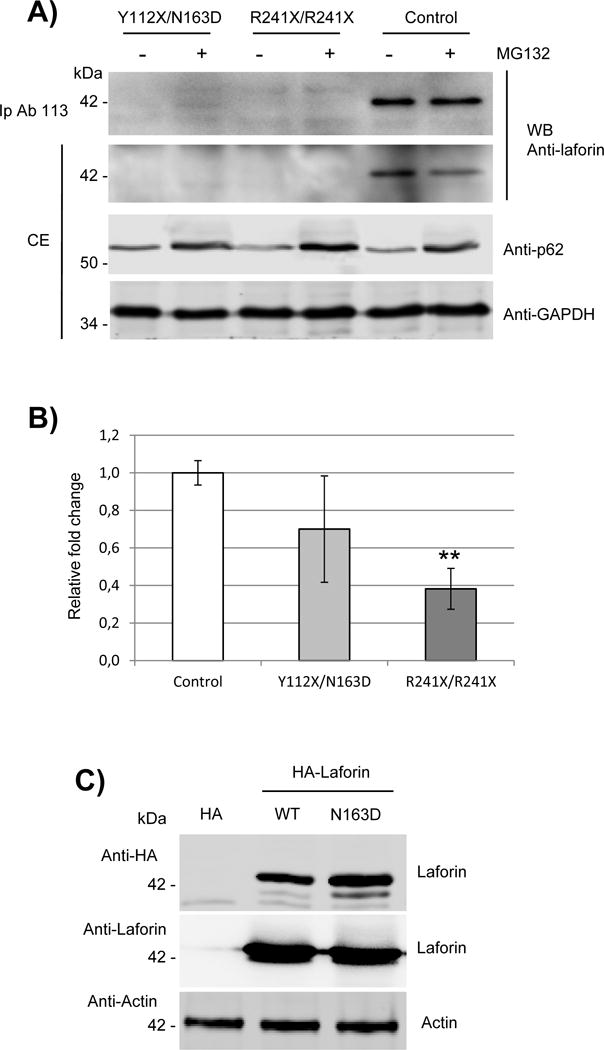
A) Western blot analysis of endogenous levels of laforin in primary fibroblasts. Crude extracts (CE, 60 μg) from primary cultures of control, EPM2A Y112X/N163D and R241X/R241X fibroblasts were analyzed by western blotting using the indicated antibodies. When indicated, samples were previously treated with 25 μM MG132 for 18h to block protein degradation. In the top panel, one mg of proteins from the clarified extracts were immunoprecipitated as in (Sherwood et al., 2013a) using anti-laforin antibody #113, and the immunoprecipitates analyzed as above. Images are representative western blots of at least three independent experiments. B) Quantitative real-time PCR analyses of the expression of EPM2A gene in primary cultures of control, Y112X/N163D and R241X/R241X fibroblasts. Gene expression was analyzed as described in Materials and Methods. The mean values of two house-keeping genes, hypoxanthine-guanine phosphoribosyltranferase (HPRT) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were used as internal controls for normalization. Values are means of three independent experiments. Bars indicate standard deviation. Differences between paired samples were analyzed by two-tailed Student’s t-tests using Graph Pad Prism version 5.0 statistical software; **P< 0.01. C) HEK293 cells were transfected with plasmids pCMV-HA (empty), pCMV-HA-Laforin or pCMV-HA-Laforin N163D. Twenty-four hours after transfection, 40 μg of crude extracts were analyzed by Western blot using anti-HA and anti-laforin antibodies. Anti-Actin was used as loading control. Images are representative western blots of at least three independent experiments.
