Abstract
Prematurity is a risk factor for respiratory syncytial virus (RSV)-associated lower respiratory tract infections (LRTIs), due to immature humoral and cell-mediated immune system in preterm newborns, as well as their incomplete lung development. Palivizumab, a humanized monoclonal antibody against the F glycoprotein of RSV, is licensed for the prevention of severe RSV LRTI in children at high risk for the disease. This study is a part of a larger observational, retrospective-prospective epidemiological study (PONI) conducted at 72 sites across 23 countries in the northern temperate zone. The aim of our non-interventional study was to identify common predictors and factors associated with RSV LRTI hospitalization in non-prophylaxed, moderate-to-late preterm infants, born between 33 weeks and 0 days and 35 weeks and 6 days of gestation, and less than 6 months prior to or during the RSV season in Bosnia and Herzegovina (B&H). A total of 160 moderate-to-late preterm infants were included from four sites in B&H (Sarajevo, Tuzla, Mostar, and Banja Luka). We identified several significant intrinsic and extrinsic factors to be associated with the risk of RSV LRTI hospitalization in the preterm infants, including: comorbidities after birth, shorter hospital stay, admission to NICU/PICU while in the maternity ward, household smoking, low maternal age, breast feeding, number of family members, and history of family/paternal atopy. Overall, our results indicated that the risk of RSV LRTI in preterm newborns can be associated with different environmental and social/cultural factors, and further research is needed to comprehensively evaluate these associations.
Keywords: Hospitalization, moderate-to-late preterm infants, respiratory syncytial virus, RSV, lower respiratory tract infection, LRTI
INTRODUCTION
Respiratory syncytial virus (RSV) is a single-stranded, negative-sense RNA virus belonging to the genus Pneumovirus of Paramyxoviridae family. RSV is highly contagious and it can cause acute respiratory tract illness in groups of all ages. In 2005, it was estimated that among children under 5 years of age at least 33.8 million cases of acute lower respiratory infection (ALRI) associated with RSV occurred worldwide, and approximately 3.4 million children required hospitalization due to severe RSV-associated ALRI. In the same study, an estimated case fatality ratio (CFR) in children younger than 5 years in developing countries was 2.1% [1]. Other studies showed that the hospitalization rate was the highest in infants younger than 6 months of age [2-4], who are at high risk of complications. Moreover, in studies of hospitalized children, RSV infection has been associated with up to 74% of bronchiolitis cases and up to 54% of pneumonia cases [3].
Epidemiological data suggested that infants with high titers of maternally acquired RSV-neutralizing antibody develop less severe RSV disease. Up to now, palivizumab, a humanized monoclonal antibody against the fusion (F) glycoprotein of RSV, has been licensed for the prevention of severe RSV-associated lower respiratory tract infections (LRTIs) in children who are at high risk for the disease [5-8]. Premature infants born at 35 weeks of gestation or less, infants with congenital heart disease or chronic lung disease are at risk for RSV-induced LRTI [9-12], as well as for re-hospitalization in the first year of life after discharge from the hospital nursery. Prematurity, along with other environmental, social and physiological factors, increases the risk of RSV-associated LRTI in infants, due to immaturity of their humoral [13] and cell-mediated immune system [14,15] and incomplete lung development [16,17].
Although epidemiological studies including single countries have been conducted to identify the risk factors for severe RSV disease [12,18,19], these results may not be generalized across different countries/populations. Taking that into account, an epidemiological study including multiple countries was conducted to identify Predictors associated with RSV hOspitalization in Non-prophylaxed, premature Infants (PONI) born between 33 weeks and 0 days and 35 weeks and 6 days of gestation. A total of 23 culturally and regionally diverse countries across the northern temperate zone were included in the PONI to determine a more universal set of risk factors for severe RSV disease in preterm infants [20]. The current epidemiological, non-interventional study is a part of the PONI study and investigates common predictors and factors associated with hospitalization due to RSV-related LRTI, in non-prophylaxed, moderate-to-late preterm infants born in Bosnia and Herzegovina (B&H) less than 6 months prior to or during the RSV season.
MATERIALS AND METHODS
Study design
The detailed description of the study design, methods and inclusion/exclusion criteria for the PONI study was published elsewhere [20]. In short, the PONI was an observational retrospective-prospective epidemiological study conducted at 72 sites across 23 countries in the northern temperate zone. The study was conducted in compliance with the protocol, Good Clinical Practice and all other applicable regulatory requirements. The study was approved by the Local Ethics Committees for all research sites independently. Data for this sub-analysis were gathered across different geographic regions located in the same area of the RSV season in B&H. The obtained data were utilized to establish a multivariable predictive model for the identification of factors highly predictive of RSV-LRTI-associated hospitalization. The enrollment period of the study was approximately 9 months (from 1 April 2013 to 28 February 2014). Data collection started on 26 September 2013 and ended on 22 July 2014 (date of the last call). At enrollment, informed consent was obtained from the subject’s legal representative. Data were retrieved from the medical records of the eligible patients. Moreover, the investigator gathered additional information from the subject’s legal representative during an interview in the hospital or by a phone call (call 1). A further contact (call 2) was made with the subject’s legal representative at the end of the RSV season (at the beginning of March) to identify the RSV hospitalization status (i.e., positive, negative, or unknown). If an infant had been hospitalized for an LRTI, the infants’ medical records were retrieved for data extraction.
Endpoints
The primary endpoint of our sub-analysis of the PONI study was hospitalization due to laboratory-confirmed RSV LRTI during the RSV season (lasted from 1 October 2013 to 30 April 2014). The primary objective was to derive predictive factors (risk factors) for RSV LRTI hospitalization, based on the observation of whether a predictive factor was present or absent in controls (i.e., infants who had not been hospitalized for LRTI, LRTInh) compared with cases (i.e., infants who had been hospitalized for RSV LRTI, RSV LRTIh). For the target RSV LRTIh group, the reference was LRTInh group. Secondary endpoints included the incidence, severity, course, and outcomes of hospitalization for RSV LRTIh and non-RSV LRTIh [nRSV LRTIh] subgroups.
Inclusion/exclusion criteria
Infants born between 33 weeks and 0 days and 35 weeks and 6 days of gestation, after 1 April 2013, with available information on LRTI hospitalization, and whose parents/legal guardians were willing to sign a patient authorization form and informed consent, were included in the study. Infants with bronchopulmonary dysplasia (BPD), chronic lung disease (CLD), hemodynamically significant congenital heart disease (hs CHD), with planned or administered RSV immunoprophylaxis at the time of inclusion, as well as those whose parent(s) had an intention to move from the local area before the end of the study period, were excluded.
Data collection
The details about data collection were published elsewhere [20]. In short, all patient data were obtained from the patient files or by interviewing the parents/legal guardians, ensuring that no data revealed the identity of patients. Data on infant characteristics and comorbidities (diagnosis of non-hs CHD, Down syndrome, immunodeficiency, cystic fibrosis, neuromuscular disorders, or other comorbidities) were collected together with perinatal data. Also, demographic data of parents and family, family history, and societal factors were obtained. During hospitalization, data on the incidence, frequency, duration and severity of LRTI hospitalization (i.e., duration of hospitalization, duration of respiratory illness, oxygen therapy, admission to pediatric intensive care unit [PICU]/neonatal intensive care unit [NICU], mechanical ventilation, and symptoms) were collected.
Statistical analyses
Statistical analysis was conducted on data of a subsample of 160 infants from B&H. Data were exported from the central PONI database to an Excel file which was imported into STATISTICA version 12 (StatSoft, Inc., OK, USA) and MedCalc Statistical Software version 15.8 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015). The sample size was underpowered but could still provide important information regarding the study objectives for the population in B&H. Numerical variables were presented as mean ± standard deviation (SD) and categorized. Categorical variables were mostly binomial and presented as such. Data related to the primary objectives were presented for the whole sample and for the two groups, LRTInh and RSV LRTIh. Results were compared using the Student’s t-test for numerical and two-sided Fisher’s exact test for categorical data, and presented as odds ratio (OR) and 95% confidence interval (CI) for the risk of RSV-associated LRTI hospitalization. Stepwise multivariable logistic regression analysis was used to identify independent predictors of RSV LRTI hospitalization. The two subgroups, RSV LRTIh and nRSV LRTIh, were compared using two-sided Fisher’s exact test with regard to the secondary objectives. A p < 0.05 was considered statistically significant.
RESULTS
A total of 160 moderate-to-late preterm infants from 4 regions in B&H were included in the study. Of them, 18 infants (11.3%) were hospitalized due to an LRTI (LRTIh): 7 (4.4%) with at least one positive test for RSV (RSV LRTIh group), 10 (6.3%) without a positive RSV test, and 1 (0.6%) with an unknown RSV status. The number of recruited infants per center was between 25 and 66 children. Overall, slightly more female (n = 83, 51.9%) than male infants (n = 77, 48.1%) were enrolled (Table 1). The number of infants born in each of the months prior to the onset of the RSV season was evenly balanced as shown in Figure 1.
TABLE 1.
Demographic and perinatal data of preterm infants with bivariate associations for RSV-LRTI-related hospitalization (N=160)
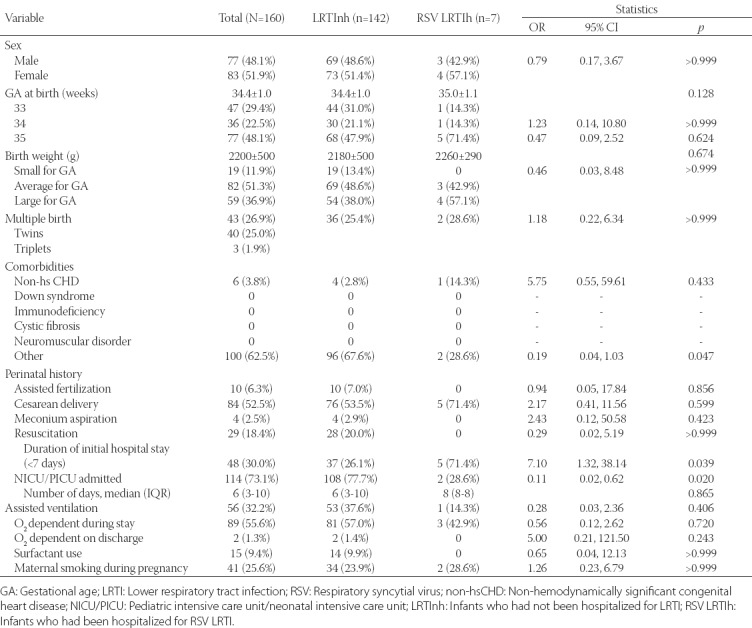
FIGURE 1.
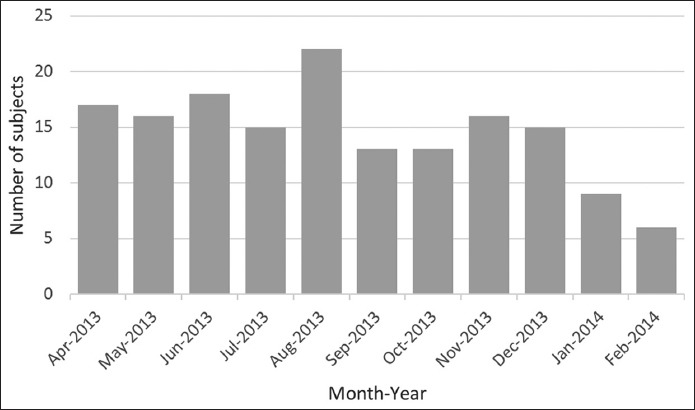
Distribution of late preterm infants enrolled in the study according to the months in which they were born, before or during the respiratory syncytial virus (RSV) season (lasted from 1 October 2013 to 30 April 2014).
Primary objective – bivariate associations
Demographic and perinatal data collected for all 160 infants at enrollment and bivariate associations for RSV-LRTI-related hospitalizations are presented in Table 1. The mean gestational age at preterm delivery was 34.4 weeks for the total population, with 29.4% (n = 47) of infants born at 33 weeks of gestational age, 22.5% (n = 36) at 34 weeks, and 48.1% (n = 77) at 35 weeks. The majority of infants in RSV LRTIh group were born at 35 weeks of gestational age (n = 5, 71.4%), but this was not significantly different compared to LRTInh group [p = 0.451] (Table 1). The mean (SD) birth weight of the total population was 2200 (500) g, without a significant difference between the groups (LRTInh vs. RSV LRTIh, p = 0.674, Table 1). Most of the infants in the total population had an average weight for gestational age (n = 82, 51.3%), 59 (36.9%) newborns were large for gestational age, and 19 (11.9%) were small for gestational age, with no significant difference between the groups (LRTInh vs. RSV LRTIh, p = 0.297, Table 1). There were 43 (26.9%) multiple birth pregnancies of which 40 were twins and 3 triplets, with no difference between the groups (LRTInh vs. RSV LRTIh, p > 0.999, Table 1). More than a half (n = 100, 62.5%) of all included infants had at least one comorbidity. Out of those 100 infants, 6 (3.8%) had confirmed non-hs CHD diagnosis, and there was no significant difference between the groups (LRTInh vs. RSV LRTIh, p = 0.433). However, a significant difference between the two groups was observed for other comorbidities (LRTInh vs. RSV LRTIh, p = 0.047, Table 1). Of the predictors for RSV hospitalization among perinatal data (i.e., assisted fertilization, type of delivery, meconium aspiration, resuscitation required, duration of initial hospital stay, admitted to the NICU/PICU prior to discharge, assisted ventilation, oxygen dependent during hospitalization or on hospital discharge, surfactant use, and maternal smoking during pregnancy) shorter hospital stay (OR for <7 days = 7.10, 95% CI 1.32-38.14, p = 0.039) and no admission to the NICU/PICU (OR = 8.71, 95% CI 1.61-47.09, p = 0.020) were associated with a significantly higher risk for RSV LRTI hospitalization, while maternal smoking during pregnancy was not associated with the risk of RSV LRTI hospitalization [p > 0.999] (Table 1).
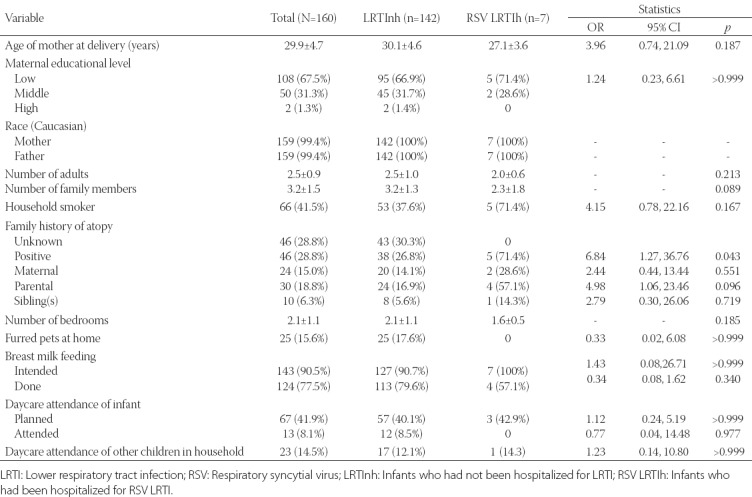
Environmental and societal data collected for all 160 infants at enrollment and bivariate associations for RSV LRTI hospitalization are presented in Table 2. The presence of a smoker in the family and low (below median) maternal age (≤28 years) were associated with a higher risk of RSV LRTI hospitalization, although they did not reach the statistical significance (OR = 4.15, 95% CI 0.77-22.16, p = 0.166 and OR = 3.96, 95% CI 0.74-21.09, p = 0.187, respectively). Low educational level of the mother was not associated with the risk of RSV LRTI hospitalization [p > 0.999] (Table 2). A higher number of family members was also not associated with the risk of RSV LRTI hospitalization; quite the opposite, the number of family members was lower in RSV LRTIh group (mean ± SD, 2.3 ± 1.8 vs. 3.2 ± 1.3 for LRTInh, p = 0.089; Table 2). Another association with the risk of RSV LRTI hospitalization was observed in terms of family atopy (OR = 6.84, 95% CI 1.27-36.76, p = 0.043), where paternal (father) atopy was associated with an increased risk of RSV LRTI hospitalization of the infant (OR = 4.98, 95% CI 1.06-23.46, p = 0.096) but not significantly, and significant results were not observed neither for maternal (OR = 2.44, p = 0.551) nor for sibling atopy [OR = 2.79, p = 0.719] (Table 2). Intention to breastfeed at enrollment was not significantly associated with a lower risk of RSV-LRTI-associated hospitalization (OR = 1.43, p > 0.999), but the actual breastfeeding lowered the risk although not significantly (OR = 0.34, 95% CI 0.08-1.62, p = 0.340; Table 2). Daycare attendance in the household did not show significant association with the risk for RSV LRTI hospitalization (p > 0.999, Table 2).
TABLE 2.
Environmental and societal data of preterm infants with bivariate associations for RSV-LRTI-related hospitalization (N=160)
Primary objective – multivariate analysis
Automated stepwise variable selection resulted in a 2-variable model depicting the admission to the NICU/PICU before discharge from the maternity ward as protective (OR = 0.11, 95% CI 0.02-0.65, p = 0.014) and family history of atopy (OR = 6.67, 95% CI 1.18-37.86, p = 0.032) as a significant risk factor for RSV LRTI hospitalization with an area under the curve of the receiver operating characteristic (AUROC) of 0.815 (95% CI 0.742-0.875, p = 0.002).
Secondary endpoints
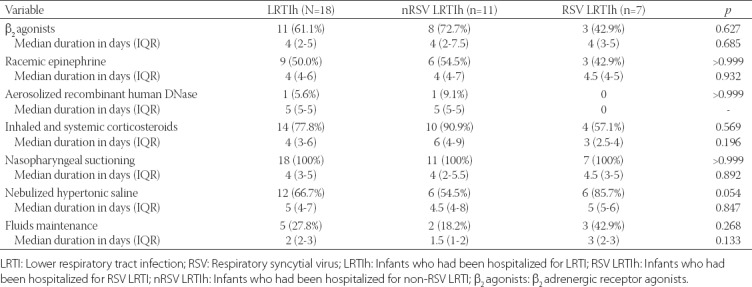
Data on LRTI hospitalization according to RSV positivity are presented in Table 3. The median duration of respiratory illness (LRTI) symptoms was 9 days, ranging from a minimum duration of 7 days to a maximum duration of 19 days, and the median hospitalization stay was 6 days with a minimum of 3 and maximum duration of 14 days, without a significant difference between RSV LRTIh and nRSV LRTIh subgroups in both cases (p = 0491 and p = 0.806, respectively; Table 3). Oxygen therapy was necessary in 7 (38.9%) infants with a median time of 3 days, with no difference in the two factors between RSV LRTIh and nRSV LRTIh subgroups (p > 0.999 and p > 0.999, respectively; Table 3). Only one infant (RSV LRTIh subgroup) required admission to the PICU/NICU for one day during the hospitalization (p = 0.667), and there was no need for mechanical ventilation in any of the LRTI-hospitalized infants (Table 3). Fever was present in 6 (33.3%) infants (all from nRSV LRTIh subgroup, p = 0.054) and cough in 16 (88.9%), with no difference in the prevalence between the subgroups (p = 0.529, Table 3). Rhinorrhea was present in 15 infants (93.8%) and was unknown in 2; the condition was more severe in nRSV LRTIh subgroup but did not reach the significance (moderate to severe, 42.9% for nRSV LRTIh vs. 0% for RSV LRTIh, p = 0.192; Table 3). Hoarseness was present in 3 infants (16.7%) and was checked as “unknown” in the majority of cases (n = 12, 66.7%; Table 3). Apnea was present in 2 infants (11.1%), 1 from each of the subgroups (p > 0.999, Table 3). Wheezing (n = 11, 61.1%) was equally distributed in both subgroups (63.6% vs. 57.1%, p > 0.999, nRSV LRTIh vs. RSV LRTIh), similarly as retractions (n = 17, 94.4%; 85.7% vs. 100%, p = 0.667, RSV LRTIh vs. nRSV LRTIh) and tachypnea [n = 17, 94.4%; 100% vs. 85.7%, p > 0.999, RSV LRTIh vs. nRSV LRTIh] (Table 3). Cyanosis was present in 4 infants (22.2%) and the proportion was comparable in both subgroups (14.3% vs. 25.0%, p > 0.999, RSV LRTIh vs. nRSV LRTIh; Table 3). The severity of the disease, graded by the investigator, was not significantly different between the two subgroups (p = 0.786), with 7 cases (38.9%) graded as mild, 7 (38.9%) as moderate and 4 (22.2%) as severe respiratory illness, in LRTIh infants (Table 3). Two infants had other viral infections (1 rhinovirus and 1 other) and 1 infant had an infection with Haemophilus influenzae [all three infants were from nRSV LRTIh subgroup] (Table 3). Medication and medical interventions during hospitalization are presented in Table 4, showing that nasopharyngeal suctioning (100%), inhaled and systemic corticosteroids (77.8%), nebulized hypertonic saline (66.7%), β2 adrenergic receptor agonists [β2 agonists] (61.1%), and racemic epinephrine (50.0%) were the most frequently used interventions with no significant difference between RSV LRTIh and nRSV-LRTIh subgroups (p > 0.05 for all parameters).
TABLE 3.
Data on hospitalization for LRTI according to RSV positivity (N=18)
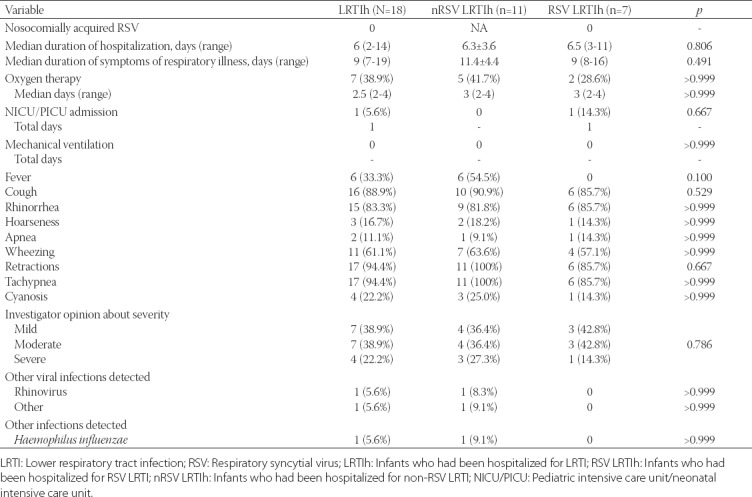
TABLE 4.
Medication and medical interventions during hospitalization according to RSV positivity (N=18)
DISCUSSION
By the age of 2 years, almost all children have an RSV infection and the reinfection is also common [21,22]; moreover, the morbidity and mortality are significantly higher in preterm compared to term infants [23-29]. Up to now, numerous extrinsic and intrinsic risk factors for RSV-associated hospitalization in early preterm infants have been identified, including: multiple gestations [30], extremely low birth weight and/or gestational age [16,17,31], intrauterine growth restriction [32], chronic lung disease of prematurity [16,17], lack of prenatal care [33], neonatal complications such as intraventricular hemorrhage, necrotizing enterocolitis, mechanical ventilation, bacteremia [34], as well as lower levels of maternal education [33] and low parental socioeconomic status [35,36]. Considering all this and its proven efficacy and safety in preterm children (≤35 weeks of gestation), palivizumab is used for the prevention of severe RSV LRTI in children requiring hospitalization and who are at high risk for the disease [5-8]. However, the indications and recommendations for the use of palivizumab in target population have been changed significantly, from the time of the first registration till now, restricting its use [37-40]. Recently, the published data for B&H showed that palivizumab provides significant protection from LRTI, and LRTI RSV hospitalization rate of 1.2% was reported for six RSV seasons, in a population in which palivizumab was used according to the updated guidelines [41,42]. The PONI study [20] aimed to identify common predictors and factors associated with RSV LRTI hospitalization in non-prophylaxed, moderate-to-late preterm infants, born less than 6 months prior to or during the RSV season, in which the American Academy of Pediatrics (AAP) does not recommend palivizumab immunoprophylaxis [40]. The study was conducted in 23 culturally and regionally diverse countries to identify a more universal set of risk factors for severe RSV disease in preterm infants (33-35 weeks of gestation) that could benefit from palivizumab immunoprophylaxis. The current sub-analysis of the PONI study, targeting B&H population, used the demographic, environmental and societal data to depict the risk factors for RSV-LRTI-associated hospitalization, for the first time in the selected regions. Although we were aware of the fact that the sample from B&H does not have enough power to show significant associations, especially for the multivariate analysis, our sub-analysis confirmed most of the significant risk factors for RSV-LRTI-related hospitalization identified in the PONI analysis [20].
In this sub-analysis, 18 infants (11.3%) were hospitalized due to an LRTI (LRTIh): 7 (4.4%) with at least one positive RSV test (RSV LRTIh), 10 (6.3%) with a negative RSV test, and 1 (0.6%) with unknown RSV status. These results are comparable to the overall PONI data [20] and to those reported by Gavin et al. [18]. It should be noted that the infant birth weight (either absolute or relative to gestational age) did not significantly affect the RSV hospitalization incidence, although it has been found that it adds to the discriminatory power of prematurity in identifying children susceptible to RSV hospitalization [42]. In the study conducted by Gavin et al., low birth weight had the highest sensitivity and the lowest specificity in predicting RSV-associated hospitalization [18].
The PONI study [20] indicated the presence of siblings (4-5 years of age) as a risk factor for RSV-associated LRTI hospitalization, however, our sub-analysis did not confirm the presence of siblings nor multiple pregnancies as the risk factors.
In this study, more than a half of the enrolled infants had at least one comorbidity of which 3.8% had a confirmed diagnosis of non-hs CHD. Even though our sample did not have enough power to statistically confirm this association, the result is in line with previous findings [1,11,16,17,21,22] and the overall PONI results [20] which showed that non-hs CHD was associated with a higher risk of RSV-associated LRTI hospitalization.
In a recently conducted study, other good predictors of RSV hospitalizations were birth stays of 7 days or more and NICU stay at birth [18]. The AAP does not list NICU stays as a risk factor for RSV prophylactic treatment. NICU stays have been used by other authors to identify high-risk infants [43], however, the bivariate and multivariate analyses in the PONI study did not find NICU stay at birth to be a significant risk factor for RSV-related hospitalization [20]. In contrast, our sub-analysis showed that shorter hospital stay (<7 days) and non-admission to the NICU were highly predictive of RSV-associated LRTI hospitalization; moreover, admission to the NICU was confirmed as a significant independent protective factor (OR = 0.11) in our multivariable model. Nevertheless, these results may have been obtained by chance due to our small sample size, or could be related to social and cultural customs where babies considered ‘sick’ are protected from the exposure to the members of extended family. On the other hand, our findings for the length of stay in the maternity ward are in line with the overall PONI results [20].
In general, more extrinsic than intrinsic factors were identified to be associated with a higher risk of RSV-associated LRTI hospitalization. If a mother smoked during pregnancy or was exposed to environmental tobacco smoke in the household following the infant’s birth, the assumption is that the infant would be exposed to tobacco smoke during the first year of life. The presence of a smoker in the family in our sub-analysis was associated with a higher risk of RSV LRTI hospitalization, confirming the overall PONI findings and the results of other studies [20,35,36]. However, contrary to the overall PONI results [20] as well as another previous study [33], in this sub-analysis maternal smoking during pregnancy and low educational level of the mother were not associated with a higher risk of RSV LRTI hospitalization. This result, however, should be interpreted with caution. Furthermore, we showed that low maternal age is associated with a higher risk of RSV LRTI hospitalization, which is in agreement with the results of PONI [20] and the study of Gavin et al. [18], and may be explained by the inexperience of younger mothers.
The presence of other children in the household is a known risk factor for RSV infection [19,24] and was also confirmed in the PONI study [20], however, in our sub-analysis it was not associated with a higher risk for RSV LRTI hospitalization.
The association between infant risk of RSV LRTI and family history of atopy has been previously shown [44], suggesting that maternal atopy is related to a higher risk of acquiring the disease [45]. Overall, our results were supportive of those findings. In addition, paternal atopy was found to be an important predictor of the risk in the PONI study [20]. However, the underlying mechanisms of how paternal atopy increases the risk of RSV-associated LRTI hospitalization, in moderate-to-late preterm infants, remains unclear.
The duration of median respiratory illness (LRTI) symptoms was 9 days in our study, of which 6 days were during the hospital stay. This is comparable to the results reported by Rodriguez et al. [46] where the median number of days with respiratory symptoms before admission was 3 (2-5) days.
Estimates suggest that every year about 1.5 million children are admitted to a hospital with severe acute LRTI in need of oxygen supplementation [1]. Oxygen therapy was necessary in less than half of our hospitalized infants, which is significantly lower than in the study conducted by Rodriguez et al, where 94.6% of patients required supplemental oxygen use [46]. Only one infant (RSV LRTIh subgroup) required admission to the PICU/NICU, for one day during the hospitalization, and there was no need for mechanical ventilation in any of our infants hospitalized for LRTI. These data suggest that the hospitalization criteria for LRTI in B&H may not be as restrictive as in some other countries and that even the infants with less severe respiratory illness are hospitalized (more than three quarters of hospitalized infants in our study were graded as mild to moderate respiratory illness by the investigator).
Although other authors found specific signs and symptoms (e.g., cough, wheezing, and retractions) to be predictive of RSV infection [1,47-50], our data did not corroborate these findings.
The limitations of the present analyses come from the low statistical power because of the small sample size and low incidence of confirmed RSV LRTI hospitalizations. This limited the possibility to identify risk/protective factors that have a smaller impact, especially in the multivariable analysis, and increased the possibility that some of the factors were associated by chance. Nevertheless, we identified several significant intrinsic and extrinsic factors (i.e., comorbidities after birth, shorter hospital stay, admission to the NICU/PICU while in the maternity ward, household smoking, low maternal age, breast feeding, number of family members, and family/paternal history of atopy) showing that the risk of RSV-LRTI-associated hospitalization can be associated with environmental and social/cultural background and that further research is needed to comprehensively evaluate these associations. Taken together, while the incidence of confirmed RSV LRTI hospitalizations was low in the present study, the clinical course and outcomes were substantial.
CONCLUSION
Our incidence of confirmed RSV-LRTI hospitalizations is in line with those reported by other studies evaluating infants born at 33 to 35 weeks gestational age. Based on the present data, comorbidities after birth were the intrinsic factor most indicative of the risk. In terms of extrinsic risk factors identified for RSV LRTI hospitalization, several significant or marginally significant associations were demonstrated, as follows: shorter hospital stay, no admission to the NICU/PICU in the maternity ward, no breast feeding, household smoking, low maternal age, number of family members, and family/paternal history of atopy. However, only two of the extrinsic factors were confirmed in the multivariable analysis, i.e., no admission to the NICU/PICU while in the maternity ward and family history of atopy. Although the incidence of confirmed RSV-LRTI hospitalizations was low in our analysis, their clinical course was notable, but nevertheless, less severe than in the other studies.
DECLARATION OF INTERESTS
Gerard Notario and Almir Zlatanovic are Abbvie employees. All the investigators have received investigator’s fee for the PONI Study. The design, study conduct, and financial support for the study were provided by Abbvie.
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. https://doi.org/10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi: 10.1056/NEJMoa0804877. https://doi.org/10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282(15):1440–6. doi: 10.1001/jama.282.15.1440. https://doi.org/10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J. 2012;31(1):5–9. doi: 10.1097/INF.0b013e31822e68e6. https://doi.org/10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 5.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–15. doi: 10.1016/s0022-3476(81)80829-3. https://doi.org/10.1016/S0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 6.Groothuis JR, Levin MJ, Rodriguez W, Hall CB, Long CE, Kim HW, et al. Use of intravenous gamma globulin to passively immunize high-risk children against respiratory syncytial virus: Safety and pharmacokinetics. The RSVIG Study Group. Antimicrob Agents Chemother. 1991;35(7):1469–73. doi: 10.1128/aac.35.7.1469. https://doi.org/10.1128/AAC.35.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meissner HC, Fulton DR, Groothuis JR, Geggel RL, Marx GR, Hemming VG, et al. Controlled trial to evaluate protection of high-risk infants against respiratory syncytial virus disease by using standard intravenous immune globulin. Antimicrob Agents Chemother. 1993;37(8):1655–8. doi: 10.1128/aac.37.8.1655. https://doi.org/10.1128/AAC.37.8.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Synagis. Synagis (palivizumab) injection. US Food & Drug Administration (FDA) approved product information. US National Library of Medicine. 2014 [Google Scholar]
- 9.Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70. doi: 10.1067/mpd.2000.110531. https://doi.org/10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen K, Stensballe LG, Bjerre J, Roth D, Fisker N, Kongstad T, et al. Risk factors for respiratory syncytial virus hospitalisation in children with heart disease. Arch Dis Child. 2009;94(10):785–9. doi: 10.1136/adc.2008.143057. https://doi.org/10.1136/adc.2008.143057. [DOI] [PubMed] [Google Scholar]
- 11.Navas L, Wang E, de Carvalho V, Robinson J. Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. J Pediatr. 1992;121(3):348–54. doi: 10.1016/s0022-3476(05)90000-0. https://doi.org/10.1016/S0022-3476(05)90000-0. [DOI] [PubMed] [Google Scholar]
- 12.Wang EE, Law BJ, Boucher FD, Stephens D, Robinson JL, Dobson S, et al. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of admission and management variation in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1996;129(3):390–5. doi: 10.1016/s0022-3476(96)70071-9. https://doi.org/10.1016/S0022-3476(96)70071-9. [DOI] [PubMed] [Google Scholar]
- 13.Aurivillius M, Oymar K, Oxelius VA. Immunoglobulin heavy G2 chain (IGHG2) gene restriction in the development of severe respiratory syncytial virus infection. Acta Paediatr. 2005;94(4):414–8. doi: 10.1111/j.1651-2227.2005.tb01910.x. https://doi.org/10.1080/08035250410023656. [DOI] [PubMed] [Google Scholar]
- 14.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195(8):1126–36. doi: 10.1086/512615. https://doi.org/10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Vecchio A, Ferrara T, Maglione M, Capasso L, Raimondi F. New perspectives in Respiratory Syncitial Virus infection. J Matern Fetal Neonatal Med. 2013;26(Suppl 2):55–9. doi: 10.3109/14767058.2013.831282. https://doi.org/10.3109/14767058.2013.831282. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham CK, McMillan JA, Gross SJ. Rehospitalization for respiratory illness in infants of less than 32 weeks’ gestation. Pediatrics. 1991;88(3):527–32. [PubMed] [Google Scholar]
- 17.Stevens TP, Sinkin RA, Hall CB, Maniscalco WM, McConnochie KM. Respiratory syncytial virus and premature infants born at 32 weeks’ gestation or earlier: Hospitalization and economic implications of prophylaxis. Arch Pediatr Adolesc Med. 2000;154(1):55–61. [PubMed] [Google Scholar]
- 18.Gavin NI, Leader S. Predictive accuracy of risk factors for RSV-related hospitalizations among infants in low-income families born at 32 to 35 weeks of gestation. Journal of Clinical Outcomes Management. 2007;14:323–31. [Google Scholar]
- 19.Blanken MO, Koffijberg H, Nibbelke EE, Rovers MM, Bont L. Dutch RSV Neonatal Network. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: A multicenter birth cohort study. PLoS One. 2013;8(3):e59161. doi: 10.1371/journal.pone.0059161. https://doi.org/10.1371/journal.pone.0059161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straňák Z, Saliba E, Kosma P, Posfay-Barbe K, Yunis K, Farstad T, et al. Predictors of RSV LRTI hospitalization in infants born at 33 to 35 weeks gestational age: A large multinational study (PONI) PLoS One. 2016;11(6):e0157446. doi: 10.1371/journal.pone.0157446. https://doi.org/10.1371/journal.pone.0157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. Respiratory syncytial virus. Red Book. 2012 [Google Scholar]
- 22.Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2012. p. 609. [Google Scholar]
- 23.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–8. doi: 10.1093/infdis/163.4.693. https://doi.org/10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. International statistical classification of diseases and related health problems (ICD-10) 10threvision. World Health Organization. 2010 [Google Scholar]
- 25.American Academy of Pediatrics (AAP), American College of Obstetricians and Gynecologists (ACOG) 7th ed. Guidelines for Perinatal Care; 2012. [Google Scholar]
- 26.Garcia CG, Bhore R, Soriano-Fallas A, Trost M, Chason R, Ramilo O, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126(6):e1453–60. doi: 10.1542/peds.2010-0507. https://doi.org/10.1542/peds.2010-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisman L. Populations at risk for developing respiratory syncytial virus and risk factors for respiratory syncytial virus severity: Infants with predisposing conditions. Pediatr Infect Dis J. 2003;22(2 Suppl):S33–7. doi: 10.1097/01.inf.0000053883.08663.e5. https://doi.org/10.1097/01.inf.0000053883.08663.e5. [DOI] [PubMed] [Google Scholar]
- 28.Meert K, Heidemann S, Abella B, Sarnaik A. Does prematurity alter the course of respiratory syncytial virus infection? Crit Care Med. 1990;18(12):1357–9. doi: 10.1097/00003246-199012000-00009. https://doi.org/10.1097/00003246-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Resch B, Gusenleitner W, Muller W. The impact of respiratory syncytial virus infection: A prospective study in hospitalized infants younger than 2 years. Infection. 2002;30(4):193–7. doi: 10.1007/s15010-002-2122-1. https://doi.org/10.1007/s15010-002-2122-1. [DOI] [PubMed] [Google Scholar]
- 30.Holman RC, Shay DK, Curns AT, Lingappa JR, Anderson LJ. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;22(6):483–90. doi: 10.1097/01.inf.0000069765.43405.3b. https://doi.org/10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 31.Simoes EA, King SJ, Lehr MV, Groothuis JR. Preterm twins and triplets. A high-risk group for severe respiratory syncytial virus infection. Am J Dis Child. 1993;147(3):303–6. doi: 10.1001/archpedi.1993.02160270065020. https://doi.org/10.1001/archpedi.1993.02160270065020. [DOI] [PubMed] [Google Scholar]
- 32.Yuksel B, Greenough A. Birth weight and hospital readmission of infants born prematurely. Arch Pediatr Adolesc Med. 1994;148(4):384–8. doi: 10.1001/archpedi.1994.02170040050008. https://doi.org/10.1001/archpedi.1994.02170040050008. [DOI] [PubMed] [Google Scholar]
- 33.Hakulinen A, Heinonen K, Jokela V, Launiala K. Prematurity-associated morbidity during the first two years of life. A population-based study. Acta Paediatr Scand. 1988;77(3):340–8. doi: 10.1111/j.1651-2227.1988.tb10658.x. https://doi.org/10.1111/j.1651-2227.1988.tb10658.x. [DOI] [PubMed] [Google Scholar]
- 34.Combs-Orme T, Fishbein J, Summerville C, Evans MG. Rehospitalization of very-low-birth-weight infants. Am J Dis Child. 1988;142(10):1109–13. doi: 10.1001/archpedi.1988.02150100103037. https://doi.org/10.1001/archpedi.1988.02150100103037. [DOI] [PubMed] [Google Scholar]
- 35.Nachman SA, Navaie-Waliser M, Qureshi MZ. Rehospitalization with respiratory syncytial virus after neonatal intensive care unit discharge: A 3-year follow-up. Pediatrics. 1997;100(6):E8. doi: 10.1542/peds.100.6.e8. https://doi.org/10.1542/peds.100.6.e8. [DOI] [PubMed] [Google Scholar]
- 36.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133(11):1135–51. doi: 10.1093/oxfordjournals.aje.a115826. https://doi.org/10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 37.McConnochie KM, Roghmann KJ, Liptak GS. Hospitalization for lower respiratory tract illness in infants: Variation in rates among counties in New York State and areas within Monroe County. J Pediatr. 1995;126(2):220–9. doi: 10.1016/s0022-3476(95)70548-1. https://doi.org/10.1016/S0022-3476(95)70548-1. [DOI] [PubMed] [Google Scholar]
- 38.American Academy of Pediatrics Committee on Infectious Diseases, Committee on Fetus and Newborn. Prevention of respiratory syncytial virus infections: Indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics. 1998;102(5):1211–6. doi: 10.1542/peds.102.5.1211. jhttps://doi.org/10.1542/peds.102.5.1211. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Pediatrics Committee on Infectious Diseases, Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112(6 pt 1):1442–6. [PubMed] [Google Scholar]
- 40.Committee on Infectious Diseases. From the American Academy of Pediatrics: Policy statements - Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124(6):1694–701. doi: 10.1542/peds.2009-2345. https://doi.org/10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 41.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–20. doi: 10.1542/peds.2014-1665. https://doi.org/10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 42.Heljic S, Maksic H, Begic H, Skokic F, Glamuzina D, Bozic T, et al. Palivizumab prophylaxis of RSV infections in Bosnia and Herzegovina. J Pediatr Neonat Individual Med. 2016;5(1):e050129. [Google Scholar]
- 43.Rietveld E, Vergouwe Y, Steyerberg EW, Huysman MW, de Groot R, Moll HA, et al. Hospitalization for respiratory syncytial virus infection in young children: Development of a clinical prediction rule. Pediatr Infect Dis J. 2006;25(3):201–7. doi: 10.1097/01.inf.0000202135.24485.f8. https://doi.org/10.1097/01.inf.0000202135.24485.f8. [DOI] [PubMed] [Google Scholar]
- 44.Joffe S, Escobar GJ, Black SB, Armstrong MA, Lieu TA. Rehospitalization for respiratory syncytial virus among premature infants. Pediatrics. 1999;104(4 Pt 1):894–9. doi: 10.1542/peds.104.4.894. https://doi.org/10.1542/peds.104.4.894. [DOI] [PubMed] [Google Scholar]
- 45.Trefny P, Stricker T, Baerlocher C, Sennhauser FH. Family history of atopy and clinical course of RSV infection in ambulatory and hospitalized infants. Pediatr Pulmonol. 2000;30(4):302–6. doi: 10.1002/1099-0496(200010)30:4<302::aid-ppul5>3.0.co;2-r. https://doi.org/10.1002/1099-0496(200010)30:4<302:: AID- PPUL5> 3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Wu CC, Chen RF, Kuo HC. Different implications of paternal and maternal atopy for perinatal IgE production and asthma development. Clin Dev Immunol. 2012;2012:132142. doi: 10.1155/2012/132142. https://doi.org/10.1155/2012/132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez DA, Rodríguez-Martínez CE, Cárdenas AC, Quilaguy IE, Mayorga LY, Falla LM, et al. Predictors of severity and mortality in children hospitalized with respiratory syncytial virus infection in a tropical region. Pediatr Pulmonol. 2014;49(3):269–76. doi: 10.1002/ppul.22781. https://doi.org/10.1002/ppul.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus - A comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79. doi: 10.1007/s12016-013-8368-9. https://doi.org/10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saha S, Pandey BG, Choudekar A, Krishnan A, Gerber SI, Rai SK, et al. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health. 2015;5(2):010419. doi: 10.7189/jogh.05.020419. https://doi.org/10.7189/jogh.05.020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durani Y, Friedman MJ, Attia MW. Clinical predictors of respiratory syncytial virus infection in children. Pediatr Int. 2008;50(3):352–5. doi: 10.1111/j.1442-200X.2008.02589.x. https://doi.org/10.1111/j.1442-200X.2008.02589.x. [DOI] [PubMed] [Google Scholar]


