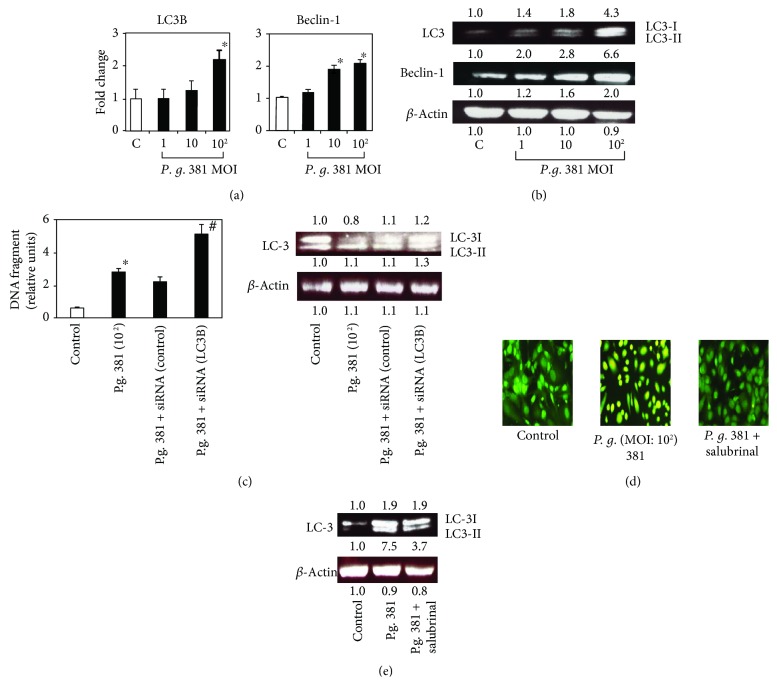
Figure 3.
P. gingivalis 381 triggers ER stress-induced autophagy in HUVEC. (a) HUVEC were treated with P. gingivalis 381 at an MOI of 1 : 1–1 : 102 for 8 h. Quantitative real-time PCR analysis was performed for LC3B and Beclin-1 relative to β-actin expression. Data are expressed as the mean ± SEM of 3 different experiments. ∗p < 0.01 versus bacteria-free control cells. (b) HUVEC were treated with P. gingivalis 381 at an MOI of 1 : 1–1 : 102 for 21 h. Whole cell lysates were subjected to SDS-PAGE, followed by Western blot analysis using antibodies to LC3 and Beclin-1. Levels of β-actin were also detected as an internal control. The density of each band was measured by densitometry, and the relative band densities were calculated by comparing the band densities with those of the control. (c) HUVEC were transfected with 50 nM of LC3B-specific siRNA or control siRNA and then stimulated with P. gingivalis 381 at an MOI of 1 : 102 for 8 h (real-time PCR) or 16 h (Western blotting). The ratio of apoptotic cells to total cells was determined using the Cell Death Detection ELISAPLUS Kit. Data are expressed as the mean ± SEM of 3 different experiments. ∗p < 0.01 versus bacteria-free control cells. #p < 0.01 versus nontransfected or control siRNA-treated cells. The expression of LC3 was analyzed by Western blotting. Lysates were then immunoblotted with anti-LC-3. Levels of β-actin were also detected as an internal control. (d) HUVEC were pretreated with an ER stress inhibitor for 1 h and then treated with P. gingivalis 381 at an MOI of 1 : 102 for 8 h. Acridine orange staining indicated that the ER stress inhibitor suppressed autophagic vacuolation induced by P. gingivalis 381. (e) HUVEC were pretreated with an ER stress inhibitor for 1 h and then treated with P. gingivalis 381 at an MOI of 1 : 102 for 21 h. Whole cell lysates were subjected to SDS-PAGE, followed by Western blot analysis using antibodies to LC3. Levels of β-actin were also detected as an internal control. The density of each band was measured by densitometry, and the relative band densities were calculated by comparing the band densities with those of the control.