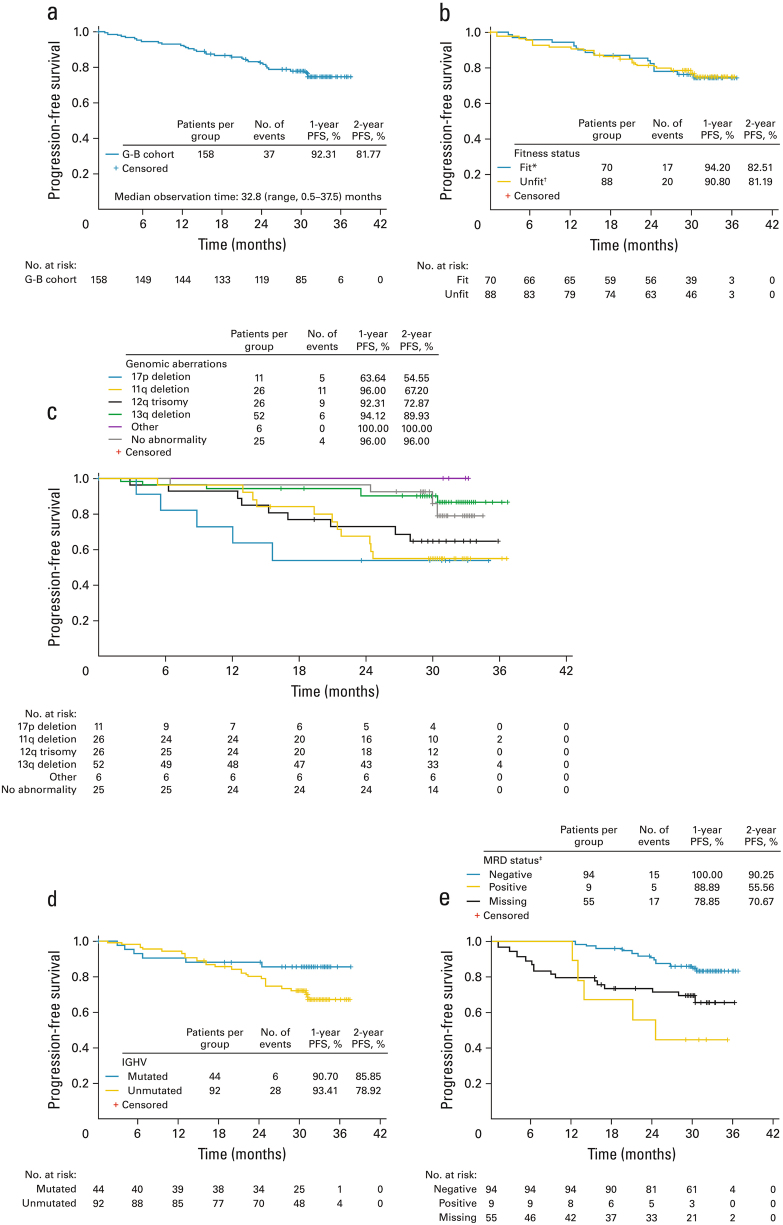
Fig. 1.
Progression-free survival in patients receiving G-B in cohort 1 of GREEN: a in the overall study population; b in fit vs unfit patients; c by genomic aberrations, according to the hierarchical model [29]; d by IGHV mutation status; and e by MRD status at final response assessment in blood (intent-to-treat population). *CIRS ≤6 and CrCl≥ 70 ml/min; †CIRS >6 and/or CrCl <70 ml/min; ‡these data should be interpreted with caution as the subgroups are based on a study outcome, not baseline characteristics. There was also a low number of events (n = 20) and small number of MRD-positive patients (n = 9). The MRD-evaluable subgroup comprised patients who did not progress or die and had an MRD result available at the final response assessment in either blood or bone marrow (n = 105). The time window for MRD assessment was 77 to 168 days after last treatment. The MRD-‘missing’ subgroup included 54 patients. In addition, 1 out of 105 patients had a result available only in bone marrow and therefore came out as ‘missing’ in the blood population (therefore the ‘missing’ group in e is n = 55 and not 54). Reasons for ‘missing’ included sample not taken (n = 20), shipment could not take place within 48 h (n = 18), measurement was outside the time window for the final response assessment (n = 9) or other reason (n = 7). Eleven patients with missing MRD status (in blood and bone marrow) progressed (n = 5) or died (n = 6) within the period since last treatment dose up to 168 days. CIRS Cumulative Illness Rating Scale, CrCl creatinine clearance, G-B obinutuzumab plus bendamustine, IGHV immunoglobulin heavy variable chain, MRD minimal residual disease, PFS progression-free survival