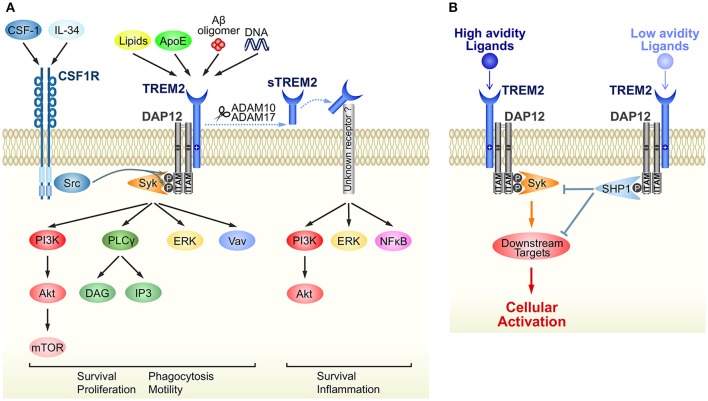
Figure 1.
Schematic representation of TREM2/DAP12 signaling in microglia. (A) Ligands and downstream signaling of TREM2/DAP12. Of the known TREM2 ligands, only ligands that highly correlate with neural diseases are shown. Upon ligand binding to TREM2, two tyrosine residues within the ITAM motif of DAP12 are phosphorylated, which recruits Syk kinase to activate downstream signaling molecules, such as ERK, PI3K, PLCγ, and Vav. Src, the main effector of CSF1R, is a kinase supposed to phosphorylate the ITAM tyrosine residues. The soluble form of TREM2, sTREM2, is generated by ectodomain shedding by ADAM 10 or 17, which activates PI3K, ERK and NFκB via an unknown receptor. Note that parts of the signaling pathways are inferred from studies of other types of myeloid cells, such as macrophages and osteoclasts. (B) A putative mechanism by which TREM2/DAP12 generates opposing signals. Upon binding of high avidity ligands to TREM2, both tyrosine residues in the ITAM motif become phosphorylated and the recruited Syk kinase activates downstream signaling molecules, as shown in (A). Conversely, in the case of low avidity ligands, only partial phosphorylation occurs and then activated SHP-1 phosphatase dephosphorylates molecules downstream of Syk signaling to inhibit cellular activation.