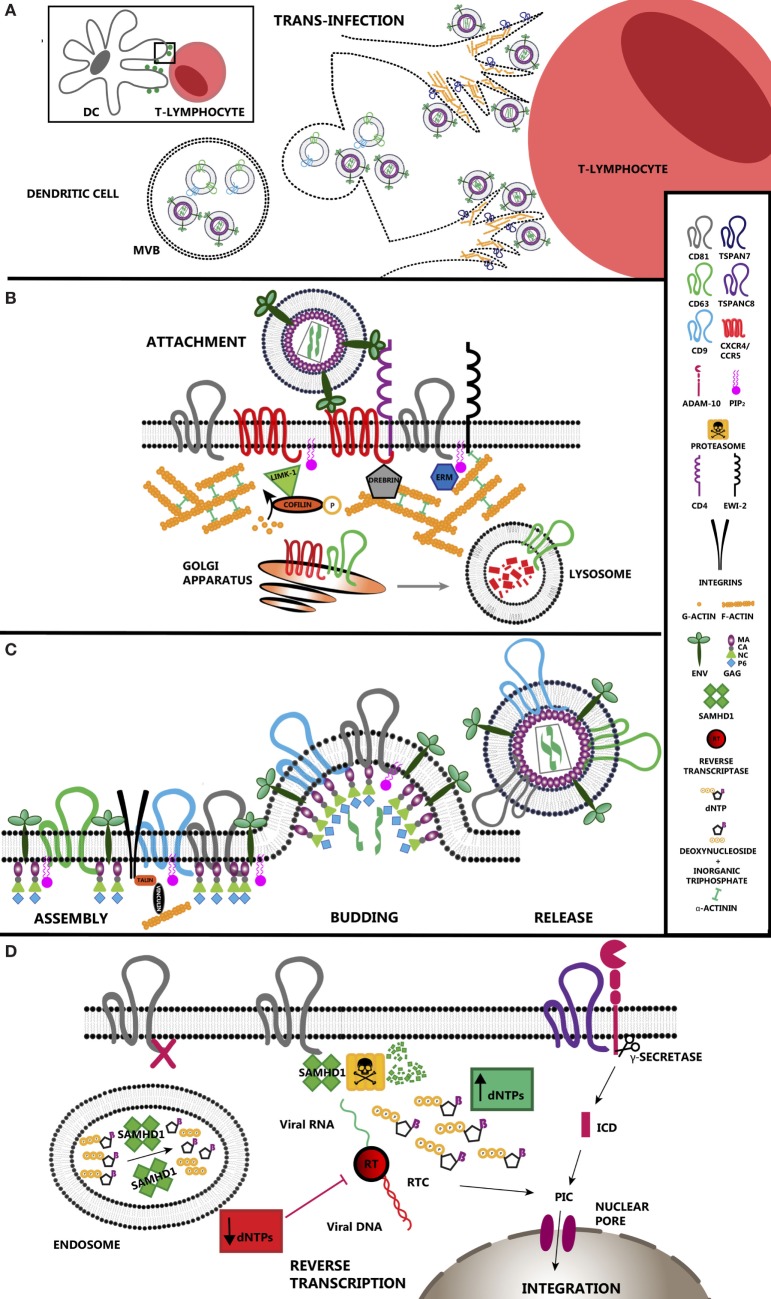
Figure 1.
Tetraspanin roles during HIV-1 infection. (A) Tetraspanins regulate transinfection of T-lymphocytes. Dendritic cells stablish contacts with T-lymphocytes during antigen presentation. HIV-1 takes advantage of immune synapses to enhance the infection of T-lymphocytes, the main target cells for the virus. This strategy is called trans-enhancement or transinfection and takes place through two different pathways. One involves the endocytosis of viral particles by DCs, which gives them access to endosomal compartments. As happens with exosomes, viral particles accumulate in multivesicular bodies that finally fuse with the plasma membrane releasing those particles together with exosomes into the intercellular space. The second pathway involves TSPAN7, which inhibits viral endocytosis and promotes formation of actin rich protrusions in DCs. In this scenario, viral particles are sequestered on the surface of these cells, allowing virus exposure and transfer to T-lymphocytes. (B) TEM regulation of HIV-1 entry. CD4 and co-receptors CCR5/CXCR4 segregate within tetraspanin-enriched microdomains (TEMs), which control their proper distribution and dynamics enhancing HIV-1 attachment efficiency and subsequent entry. CD63 regulates the expression of CXCR4 on the cell surface by stimulating its degradation through the lysosomal pathway. Env binding to its receptor and co-receptor brings them closer and triggers several intracellular pathways where actin polymerization is the main response. Active LIMK1 phosphorylates and inactivates cofilin, stimulating actin polymerization. Proteins such as moesin or α-actinin have a structural function as they link receptors and tetraspanins to the subcortical actin network. Other proteins such as drebrin control the stability of the actin web. TSPAN7 is also a positive regulator of actin polymerization, although the effectors downstream have not been addressed yet. (C) HIV-1 assembly occurs at TEMs. Viral protein Gag interacts with the inner leaflet of the plasma membrane via its myristoylation, which increases the affinity for cholesterol-enriched areas. Gag also interacts with the positively charged PIP2 and the inner loop of different tetraspanins such as CD81 and CD82. Gag induces CD9 clusterization. However, there is no direct evidence indicating an essential requirement for tetraspanins during HIV-1 budding. Recruitment of all these components into restricted areas may involve the presence of the subcortical actin web for their stabilization, where talin and vinculin would act as a link. (D) HIV-1 reverse transcription (RT) is regulated by tetraspanins. SAMHD1 is a negative regulator of viral RT as it decreases the concentration of deoxynucleotide triphosphates available in the cell. CD81 regulates SAMHD1 activity by stimulating its degradation via proteasome. CD81 depletion induces the relocalization of SAMHD1 inside early endosomes. ADAM-10 activity is regulated by tetraspanin TSPANC8 subfamily. The resulting intracellular domain when cleaved by a γ-secretase has been identified recently as a component of the PIC. When RT is completed, viral DNA is transported into the nucleus where it integrates in the cell genome.