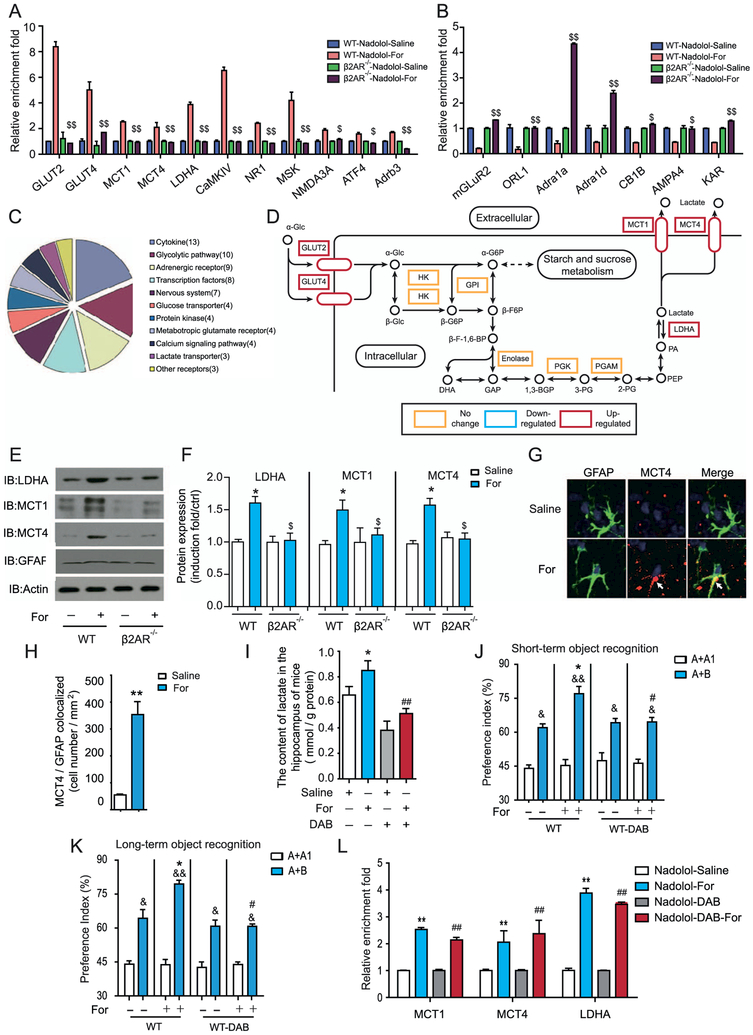
Figure 2.
Activation of β2-adrenergic receptors (β2ARs) promotes astrocyte-neuron lactate transport, which is required for increased learning ability. (A, B) Quantitative reverse transcriptase polymerase chain reaction (QRTPCR) analysis of the messenger RNA (mRNA) transcription profiles of genes important for learning and memory after formoterol (For) stimulation for 3 days in wild-type (WT) or β2AR−/− mice. (A) After continuous formoterol administration, mRNA levels of the genes showed an increase in the WT but not in the β2AR−/− mice. (B) mRNA levels of the genes showed a decrease in WT mice but were abolished in β2AR−/− mice. Expression levels were normalized to actin levels. (C) The graph represents the clustering of the 69 important learning and memory genes that were examined (in [A]) into subfunctional groups. (D) Activation of β2ARs in the hippocampus upregulates the expression of several key genes in the lactate metabolism and transport subnetworks. Gene transcription with significant increases is shown in the red rectangle, and genes with no significant changes are shown in the yellow rectangle. (E, F) Activation of β2ARs by formoterol increased protein levels of lactate dehydrogenase A (LDHA), monocarboxylate transporter 1 (MCT1), and MCT4 in the hippocampus of WT mice but not β2AR−/− mice. (E) Representative Western blots for MCT1, MCT4, and LDHA in the hippocampus from WT or β2AR−/− mice. (F) Bands from Western blots (performed in triplicate as a minimum) were quantified and expressed as induction folds over the control (E) (*p = .037, p = .04, p = .016; $p = .021, p = .048, p = .027). (G) Representative immunostaining image of MCT4 in the hippocampus with/without formoterol treatment for 3 days. (H) Statistical analysis and a bar graph represent the colocalization of MCT4 expression with glial fibrillary acidic protein (GFAP) in (G) and Supplemental Figure S9. n = 6 mice per group; four random areas were picked out from each hippocampus section, and five sections were randomly selected from each mouse. The colocalization of MCT4 with the marker of the astrocytes (GFAP) was significantly increased after formoterol treatment. (I) Formoterol administration increased lactate levels in the hippocampus, which is blocked by the inhibitor of glycogen phosphorylation DAB. (J, K) Improved object preference in recognition memory after formoterol administration was abolished by 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) treatment (n = 10 for each group: [J] *p = .036, #p = .036, &p = .033, &&p = .006, &p = .03, p = .04; [K] *p = .035, #p = .04, &p = .03; &&p = .0029; &p = .04, p = .04). (L) QRTPCR analysis of mRNA transcription profiles of genes important for learning and memory after saline, formoterol, DAB, and formoterol and DAB stimulation for 3 days in WT mice. Results show that DAB application has no significant effect on mRNA levels of MCT1, MCT4, and LDHA. Data were normalized to actin levels. (A–L) All animal experiments were performed with at least six animals in each group. *p < .05; ***p < .005; formoterol-treated animals were compared with control vehicle-treated animals. #p < .05; DAB-treated animals were compared with control vehicle-treated animals. $p < .05, $$p < .01; β2AR−/− mice were compared with their WT littermates. &p < .05, &&p < .01; preference indexes before and after training were compared. All data were analyzed with one- or two-way analysis of variance. Adra1a, adrenergic receptor, alpha 1a; Adra1d, adrenergic receptor, alpha 1d; Adrb3, adrenergic receptor, beta 3; AMPA4, glutamate receptor, ionotropic, alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate receptor 4; ATF4, activating transcription factor 4; CaMKIV, calcium/calmodulin-dependent protein kinase IV; CB1B, cannabinoid receptor 1; ctrl, control; GPI, glucose phosphate isomerase; HK, hexokinase; IB, immunoblot; KAR, voltage-sensitive potassium/chloride channel; mGLuR2, metabotropic glutamate receptor 2 (G-protein-coupled receptor type); MSK, mitogen and stress activated protein kinase; NMDA3A, N-methyl-D-aspartate receptor 3A (glutamate receptor, ion channel type); NR1, ionotropic, N-methyl-D-aspartate receptor channel, subunit zeta-1 (glutamate receptor, ion channel type); ORL-1, opioid receptor-like 1; PGAM, phosphoglycerate mutase; PGK, phosphoglycerate kinase.