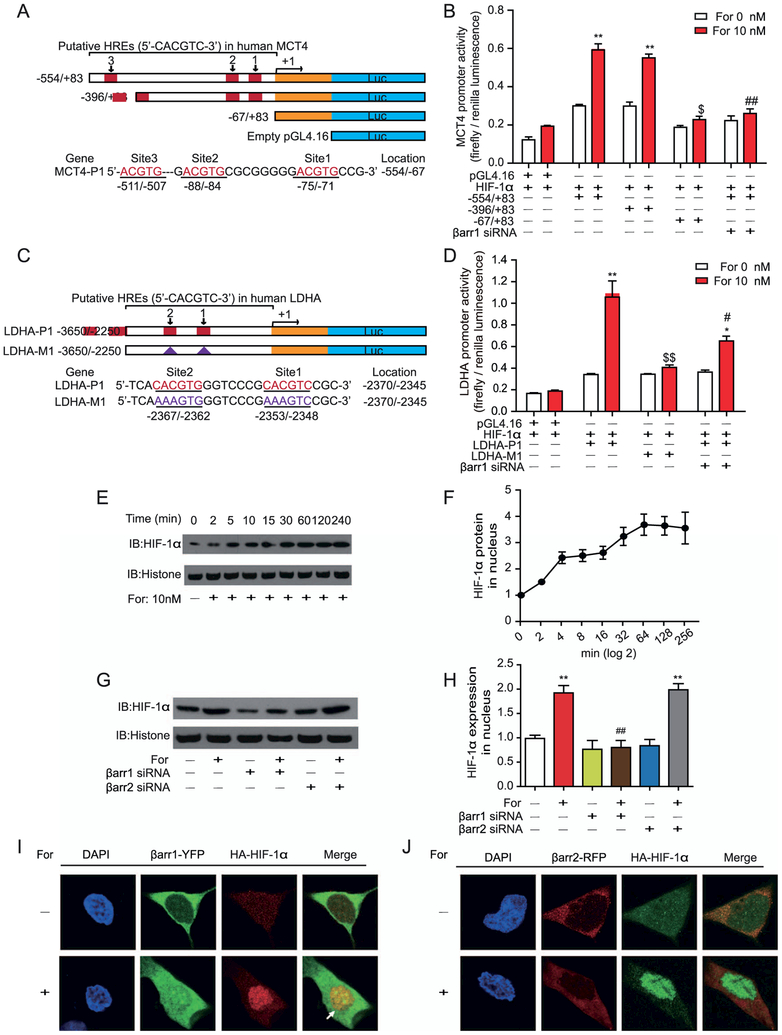
Figure 6.
Activation of β2-adrenergic receptor (β2AR) regulates monocarboxylate transporter 4 (MCT4) and lactate dehydrogenase A (LDHA) transcription through translocation of hypoxia-inducible factor-1α (HIF-1α) into the nucleus. (A, B) Deletion analysis of human MCT4 promoters on the 5′-flanking sequences. (A) The scheme depicts promoter sequences with different hypoxia-response elements (HREs) that were cloned into a pGL4.16 vector, and the firefly luciferase reporter gene was expressed to determine promoter activity. (A) HRE sites are defined as 5′-ACGTG-3′, and the locations are indicated. Serial 5′ deletions of the full-length MCT4 promoters (−554/83) were generated by polymerase chain reaction. The yellow rectangle represents the exon. (B) β2AR–β-arrestin-1 (βarr1) signaling regulated MCT4 transcription through the binding of HIF-1α to the promoter sites. Formoterol (For)-induced wild-type and truncated MCT4 promoter activity were assayed by luciferase activity. The contribution of βarr1 was monitored by βarr1 small interferring RNA (siRNA). (C) The scheme represents the HRE binding site on the LDHA promoter. Two HIF-1α binding sites were identified upstream of the transcriptional starting site. Mutations of the HIF-1α binding sites were designated as LDHA-M1. (D) β2AR-βarr1 signaling regulated LDHA transcription through HIF-1α. Formoterol-induced wild-type or mutated LDHA promoter activity was assayed by luciferase activity. Mutations of the HIF-1α binding site or knockdown of βarr1 significantly decreased LDHA transcriptional activation. (E, F) Nucleus HIF-1α levels at different time points were (E) demonstrated by Western blots and (F) subjected to statistical analysis. Cells were treated with 10 nmol/L of formoterol for the indicated time. (G, H) βarr1 is required for the formoterol-induced nuclear translocation of HIF-1α. (G) Representative Western blot images and (H) statistical analysis. Cells were transfected with control siRNA, βarr1 or βarr2 targeting siRNA before formoterol treatment (**p = .0022, p = .0027; ##p = .0033). (I, J) Localizations of HIF-1α and βarr1/βarr2 with or without formoterol stimulation. Confocal images show (I) βarr1, HIF-1α (green and red), and (J) βarr2, HIF-1α (red and green) from cells treated with dimethyl sulfoxide (DMSO) (control) or 10 nmol/L of formoterol. There were few colocalizations between βarr1/βarr2 and HIF-1α in cells treated with DMSO. Formoterol treatment induced the colocalization of HIF-1α and βarr1. (A–J) At least three independent experiments were performed for all statistical analyses. *p < .05; formoterol-treated samples were compared with control vehicles. #p < .05; ##p < .01; β-arrestin siRNA was compared with control siRNA, and mutated promoters were compared with wild-type promoters. $p < .05; $$p < 0.01; mutations/deletions of MCT4/LDHA promoters were compared with wild-type MCT1/LDHA promoters. (E, F) Representative Western blots from at least three independent experiments are shown and quantified. DAPI, 4′,6-diamidino-2-phenylindole; HA, hemagglutinin antigen; IB, immunoblot; RFP, red fluorescent protein; YFP, yellow fluorescent protein.