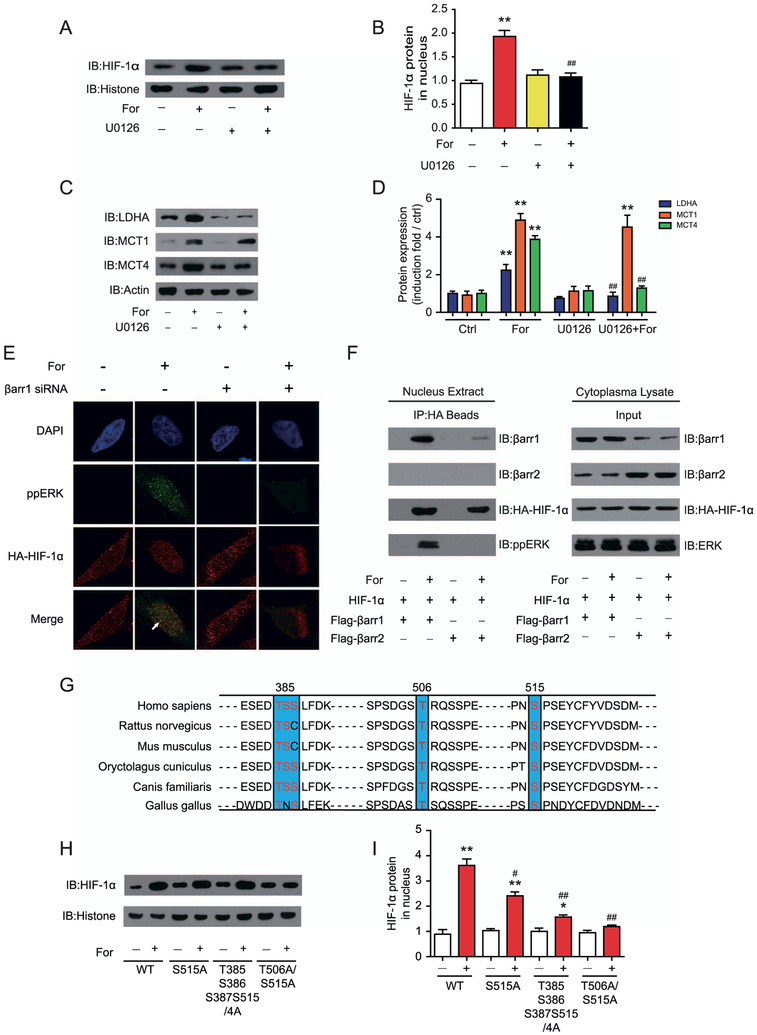
Figure 7.
Activation of β2-adrenergic receptor (β2AR) leads to formation of a β-arrestin-1 (βarr1)-mitogen-activated protein kinase (ERK)-hypoxia-inducible factor-1α (HIF-1α) ternary complex and phosphorylation of HIF-1α at the T506 and S515 positions, which are required for HIF-1α retention in the nucleus. (A, B) ERK activation is required for the translocation of HIF-1α after β2AR activation. U251 cells were treated with or without the specific MEK inhibitor butanedinitrile, 2,3-bis[amino[(2-aminophenyl)thio]methylene] (U0126) before formoterol (For) treatment. (A) Representative Western blots for nucleus HIF-1α levels and (B) the statistical analysis (**p = .0035; ##p = .0041). (C, D) Effects of the MEK inhibitor U0126 on formoterol-induced lactate dehydrogenase A (LDHA), monocarboxylate transporter 1 (MCT1), and MCT4 expression in U251 cells. (C) Representative Western blots, and (D) the statistical analysis (**p = .0031, p = .0017, p = .0026, p = .0021; ##p = .0045, p = .0041). (E) Colocalization of phospho-ERK (ppERK, green) and HIF-1β (red) in the nucleus after formoterol stimulation. Cells in the left panel were preincubated with control (Ctrl) small interferring RNA (siRNA), and cells in the right panel were preincubated with βarr1 siRNA. (F) Nuclear extracts of astrocytes cotransfected with hemagglutinin antigen (HA)-HIF-1α and Flag-βarr1/2 were prepared with or without formoterol treatment for 1 hour. Formation of the βarr1-ERK-HIF-1α ternary complex was analyzed by the immunoprecipitation of HA-HIF-1α, and βarr1, βarr2, and pp-ERK were detected in the immunoprecipitated complex with specific antibodies. (G) Sequence alignment of the potential ERK phosphorylation sites in HIF-1β between different species. (H, I) Effects of HIF-1β mutations on formoterol-induced HIF-1β translocation. Wild-type (WT) HIF-1α or HIF-1α S515A, T385S385S387S515/4A, and T506A/S515A were transfected into U251 cells. (H) After formoterol stimulation for 1 hour, the nucleus extract was prepared, and the presence of HIF-1α in the nucleus was examined by Western blot. (I) Statistical and bar graph representation of Western blot bands from (H) (**p = .0021, p = .004; *p = .015; #p = .044, ##p = .0068, p = .0029). (A–I) Representative Western blots from at least three independent experiments are shown. At least three independent experiments were performed for all statistical analyses. *p < .05; formoterol-treated samples were compared with control vehicle samples. #p < .05; ##p < .01; U0126-treated cells were compared with control cells, and cells transfected with the HIF-1β mutant were compared with cells transfected with the HIF-1β WT. DAPI, 4′,6-diamidino-2-phenylindole; IB, immunoblot.