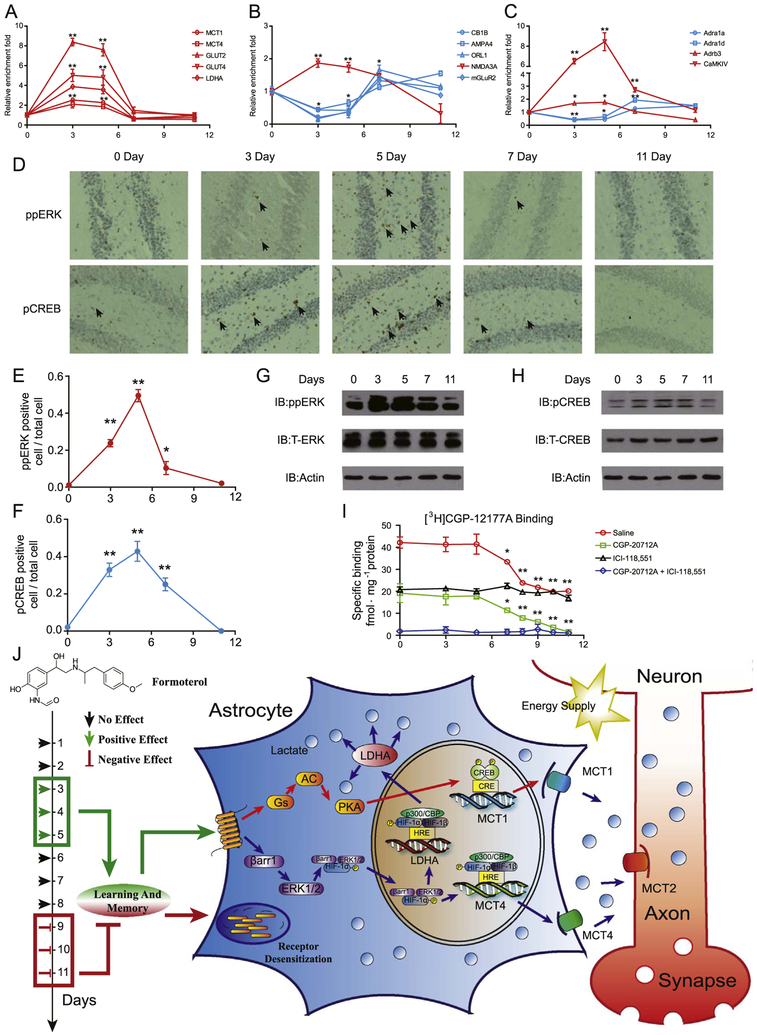
Figure 8.
Prolonged activation of β2-adrenergic receptor (β2AR) causes desensitization. (A–C) Quantitative reverse transcriptase polymerase chain reaction analysis of messenger RNA levels of genes important for β2AR-dependent signaling networks after formoterol stimulation for 3, 5, 7, or 11 days. Genes enriched in (A) the lactate metabolism and transport network, (B) the glutamate receptor-synaptic long-term potentiation signaling network, and (C) the calcium signaling network. Red, genes that exhibited an increase on days 3 and 5 compared to the initial date; blue, genes that exhibited a decrease on days 3 and 5 compared to the initial date. (D) Representative immunohistochemistry image of phospho-mitogen-activated protein kinase (ppERK) (upper panel) and phospho-cyclic adenosine monophosphate response element binding protein (pCREB) (lower panel) in the hippocampus after formoterol treatment for 3, 5, 7, and 11 days. (E, F) Statistical analysis and line chart representing the percentage of cells with positive staining of ppERK or pCREB in (D) (six sections were randomly selected from each mouse. n = 6 for each group of mice.). (G, H) Western blot of ppERK or pCREB in the hippocampus from the wild-type mice treated with formoterol for 3, 5, 7, or 11 days (upper panel). Representative blots from at least three independent experiments are shown. Protein levels of total ERK (T-ERK) and total CREB (T-CREB) were also examined with specific antibodies (middle panel). (I) β2AR protein levels in the plasma membrane fraction of the hippocampus from wild-type mice treated with formoterol for 3, 5, 7, or 11 days. Then, 100 nmol/L of [3H]CGP-12177A was used for radioligand binding experiments to measure βAR protein levels on the plasma membrane in the hippocampus. Next, 10 μmol/L of CGP-20712A (specific β1AR antagonist) or 10 μmol/L of ICI 118,551 (specific β2AR antagonist) was used to block specific β1AR or β2AR binding, respectively (saline: * p = .0288; **p = .0098, p = .0089, p = .0080, p = .0074; CGP-20712A: *p = .0350; ** p = .0099, p = .0081, p = .0076, p = .0052). (J) Administering a suitable dose of the clinical drug formoterol improves learning and memory in treatment lasting a few days (3–5 days), whereas administration times longer than 1 week are harmful (9–11 days). The beneficial effect of formoterol occurs through β2AR activation. Transcriptional profile analysis and cellular studies revealed that β2AR activation promoted lactate metabolism through both the Gs-protein kinase A (PKA)-CREB-MCT1 and β-arrestin-1-ERK-HIF-1α-MCT4/LDHA pathways. The lactate-shuttling cycle between astrocytes and neurons is required for improved cognitive function stimulated by formoterol treatment in the early days. In contrast, prolonged formoterol administration caused desensitization of β2AR and decreased cognitive ability. (A–I) * p < .05; ** p < .01; formoterol-treated samples were compared with nontreated (0 day) samples. Adra1a, adrenergic receptor, alpha 1a; Adra1d, adrenergic receptor, alpha 1d; Adrb3, adrenergic receptor, beta 3; AMPA4, glutamate receptor, ionotropic, alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate receptor 4; CaMKIV, calcium/calmodulin-dependent protein kinase IV; CB1B, cannabinoid receptor 1; GLUT2, glucose transporter 2; IB, immunoblot; LDHA, lactate dehydrogenase A; MCT1, monocarboxylate transporter 1; mGLuR2, metabotropic glutamate receptor 2 (G-protein-coupled receptor type); NMDA3A, N-methyl-D-aspartate receptor 3A (glutamate receptor, ion channel type); ORL1, opioid receptor-like 1.