Abstract
Titanium (Ti) plays a predominant role as the material of choice in orthopaedic and dental implants. Despite the majority of Ti implants having long-term success, premature failure due to unsuccessful osseointegration leading to aseptic loosening is still too common. Recently, surface topography modification and biological/non-biological coatings have been integrated into orthopaedic/dental implants in order to mimic the surrounding biological environment as well as reduce the inflammation/infection that may occur. In this review, we summarize the impact of various Ti coatings on cell behaviour both in vivo and in vitro. First, we focus on the Ti surface properties and their effects on osteogenesis and then on bacterial adhesion and viability. We conclude from the current literature that surface modification of Ti implants can be generated that offer both osteoinductive and antimicrobial properties.
Keywords: Titanium implant, topography, osteogenesis, bacterial adhesion, surface coating
Introduction
Titanium (Ti) and its alloys are commonly used materials in orthopaedic and dental implants due to their mechanical and chemical properties; these include high strength to weight ratio and high yield and fatigue strength along with a relatively low Young’s modulus counteracting the effects of stress shielding. An instantaneously forming passive oxide layer leads to corrosion resistance and biocompatibility.1–5 Moreover, Ti is amenable to alterations in physical and chemical properties, including changing the surface oxide composition, thickness and topography, together making Ti a suitable material for enhancement via surface modification.6 The biocompatibility of Ti and its alloys are related to the capacity of the Ti oxide layer to react with water ions and serum proteins as well as the resistance to corrosion that provided by the oxide layer.7–10
Scaffold surface features need to be biocompatible, bioactive and perhaps biodegradable as they are replaced by natural tissue during the regenerative process. Replicating the key structures of the extracellular matrix (ECM) and providing stem cell environments are powerful bioactive strategies that material scientists can copy and exploit.11 Although Ti materials have many favourable properties, there are known potential shortcomings. For example, aluminium in Ti alloys may be associated with neurological disorders.12 In addition, intra-articular injection of Ti dioxide (TiO2) nanoparticles in rats has been noted to cause toxicological effects in lungs with follicular lymphoid hyperplasia and inflammatory cells aggregated around the bronchia.13 Moreover, ionic Ti may have a mutagenic effect on cells either directly by damaging DNA via free radicals or indirectly by inhibiting the DNA repair14 and may also induce some allergic reactions.4,15 The biological response to orthopaedic and dental implants is determined by the physical and chemical features of the implant surface. These include surface topography, surface free energy, oxide thickness and oxide composition. The interaction between cells and the interface will be affected by one or more of these factors and any change in one will affect the other parameters.16–19 Surface topography has the ability to regulate the cell behaviour in a reproducible manner.20 Furthermore, advances in topographical fabrication are making nanoscale topographical features achievable in a large scale on more complex materials (traditionally only flat surfaces and small surface areas have been able to be patterned at the nanoscale).21 The use of topography to guide mesenchymal stem cells (MSCs) may, in fact, play a key role in bone tissue engineering as, unlike chemical and mechanical alterations, topographical modifications do not affect the bulk properties of materials and orthopaedic materials need to be able to support load. The stem cells’ ability to adhere and spread into specific surfaces has shown a dramatic effect in cellular development.22 Osseointegration is the direct contact between bone and the implant, with histological evidence suggesting that new bone is forming around the inert object. The quality and amount of osseointegrated bone around the implant, in addition to other factors such as the degree of inflammation, an excessive force, may affect their stability and consequently their failure rates.23 Osseointegration and subsequent mineralization is dependent on the initial adhesion of fibrin in blood-mediated osseointegration of osteoblasts or MSCs onto the implant surface.12,24,25 Failure to achieve osseointegration will lead to premature implant failure and this integration is required to be maintained throughout the implant’s lifespan to ensure longevity26 although patient and surgical related technical/environment factors may also contribute to failure.27 For instance, among patient factors, male gender, smoking, autoimmune disease and penicillin allergy showed a trend towards greater failure rates.28–30 Late-stage failure tends to occur as a result of implant overloading, wear and peri-implantitis.31
Moreover, implant infection is the most serious issue after surgery. Biomaterial centred infections (BCI) and prosthetic implant infections (PIIs) have a significant contribution in prosthetic implant failure and aseptic loosening32,33 with the average rate 2%–5%.34 Host defence mechanisms and current antibiotic treatments become ineffective when bacterial biofilms build up.35,36 However, Ti is generally considered a very safe and highly biocompatible material that has had extensive clinical use for many decades.
Surface properties
Albrektsson and Wennerberg37 subdivided the implant surface quality into three categories: mechanical properties, topographical properties and physicochemical properties. They conclude that these characteristics are related and by altering any of these groups, the others will also be affected. With Ti, altering the mechanical properties within the physiological range is hard to achieve and so chemistry and topography are the main focus.37
Biological (in the bone forming sense) materials can be roughly classified into three categories: (1) biotolerant materials where a thin fibrous tissue interface is formed; (2) bioinert materials, like Ti, that can have direct bone contact under osteopermissive conditions; and (3) bioactive materials like calcium phosphate ceramics which can have high degree of direct contact bond with the surrounding bone which is believed to be due to the presence of free calcium and phosphate at the implant interface.38 More recently, these have been re-categorized as first generation (structural, biocompatible), for example, Ti, second generation (bioactive), for example, hydroxyapatite (HA), bioglass and third generation (reproducible molecular control), for example, nanotopography.39
Biocompatibility is important to prevent an immune response and foreign body reaction when the material is introduced into the human body.40 The primary interaction between material and host starts with a thin interface zone, which includes rapid protein adsorption and interaction with the connective tissues. This first interaction is controlled by physical and chemical properties such as roughness, structure, defects and oxide thickness and is critical for long-term implant success.6,41
In this review, we will discuss the importance of Ti surface properties on the bioactivity of implants.
Surface wettability
Wettability is measured by contact angle measurement, usually of water, at the solid/liquid interface while surrounded by a gas phase or another liquid phase and provides gross surface characterization. A low contact angle of less than 90° indicates a hydrophilic surface; the liquid will subsequently spread over the surface. A large contact angle of more than 90° signifies that the surface is hydrophobic leading to droplet of liquid forming on the interface. However, this reaction is controlled by the molecular interaction between the different phases.42,43 Other factors such as surface tension and surface energy are also determined by surface wettability.44
Liquids can interact with two different types of solid surfaces: high and low energy solid surfaces. Metals, glass and ceramics are examples of solid surfaces with high energy (hard solids) where molecular liquids achieve complete wetting on these solids. The weak solids like fluorocarbons and hydrocarbons have a low energy, where liquid molecules would take a very low energy to break them providing a complete or partial wetting depending on the liquid chosen.42,45
However, increasing the surface wettability may enhance the fibrin adhesion and provide contact guidance for osteoblast migration along the surface.46 Moreover, any change in surface wettability will affect protein adsorption which consequently changes cell adhesion through integrins and non-integrin receptors.47
Surface chemistry
The surface chemistry is an important factor in improving the osseointegration. The chemistry of the surface will dictate the interaction of cells with surface proteins in a number of ways: (1) chemical adsorption including covalent bonds and ionic bonds, (2) electrostatic forces found in electrokinetic potential or zeta potential, (3) hydrogen bonds involved in hydrophilic groups, (4) hydrophobic interaction and (5) van der Waals forces.48 It is known, for example, that osteoblasts are sensitive to subtle differences in surface chemistry.49
For instance, the fluorine-modified implant surface accelerated osseointegration in the early stage of healing which improved the growth of peri-implant tissue, enhanced the adhesion strength and influenced the osteogenesis gene level.50,51 Also, through control of the oxide chemistry and surface charge, charged antimicrobials may be applied to help fight potential infections.52 Shibata et al.53 showed that the TiO formed on the Ti–Cl surface enhanced cell extension and cell growth through a larger adsorption of fibronectin (FN) compared with control, while the TiCl3 contributed to the antibacterial activity of Ti–Cl.
Oxide thickness
Ti, in the presence of air or water, reacts with oxygen to form a protective, chemically stable oxide layer that has the capability to reform immediately after any disturbance. This oxide layer gives the implant increased corrosion resistance, a low rate of ion release and good biocompatibility via plasma protein interactions (e.g. fibrin, fibronectin, vitronectin).6,54,55 An interesting characteristic of the oxide layer is that it can be induced to provide antibacterial behaviour through light excitation without affecting mammalian cell cytocompatibility.56 For example, light irradiation of amoxicillin gold nanoparticle composites (amoxi@AuNPs) showed a photo-antimicrobial effect on Staphylococcus aureus.57 Furthermore, studies have shown the importance of the oxide layer thickness in bone formation on implants where bone contact may improve via increasing the oxide layer thickness.58
Surface roughness and nanostructure
Surface roughness has a vital role in bone healing and enhancing the biomechanical properties by increasing the mechanical retention (interdigitation) and providing good stress distribution. Surface roughness can be divided into three levels: macro-roughness (Ra scale around 10 µm), micro-roughness (Ra scale around 1 µm) and nano-roughness (Ra scale < 200 nm). Ra is an arithmetic average of the absolute values of vertical deviations from a mean plane.46,59
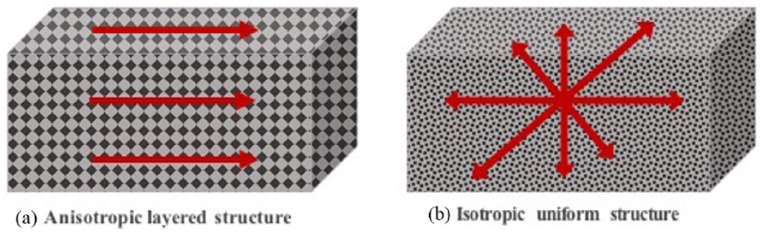
Implant roughness can also be classified depending on feature morphology such as concave textures, for example, HA coating/titanium plasma spraying and convex textures, for example, etching and blasting treatments.60 Another classification of implant roughness is the orientation of surface irregularities such as isotropic surfaces where topographies are independent of direction and anisotropic surfaces that have a clear direction4,61 (Figure 1).
Figure 1.
The difference between anisotropic and isotropic surfaces. (a) Anisotropic surfaces have clear directionality, differ considerably in roughness and the materials properties are not the same at all points or directions. (b) Isotropic surfaces have the same topography independent of measuring direction and the physical property is the same at any point/direction through the material.
In cases of poor bone quality and reduced bone volumes, surface roughness is often used in clinical situations to help accelerate and enhance osseointegration and bone interlocking.46 Previous studies have shown that the optimal Ra needs to be around 1–1.5 µm; otherwise, the implant fixation would be weakened.62 Increasing surface roughness can, however, via increased surface area, increase the potential of microbial colonization and provide a shelter to bacteria, hence avoiding removal by antibiotics.26,63 However, previous studies have shown that surface roughness below 0.2 µm was less likely to promote bacterial adhesion as most bacteria are larger in size.64,65 The above studies, however, really only deal with topography for mechanical integration rather than cellular integration (osseoinduction). Surface topography can indeed influence the rate at which bone is formed next to the surface, and perhaps more so than the surface oxide thickness or microstructure66 as will be further discussed.
There has been some success with improving secondary cellular fixation, using topographical modifications. Most techniques that are used to produce nanotopography on Ti such as sand-blasting,67 acid etching,68,69 cluster deposition,70 layer-by-layer assembly71 and anodization72 generate less defined features, lacking precise control and tunability of the topographies.73 While such nanoscale features may lead to changes in the cell number, size, focal adhesion arrangements, cytoskeletal and nucleoskeletal organization, reproducible changes may be hard to achieve because of batch to batch variations. More precise control over nanofeatures traditionally requires lithographical techniques that are hard to use with materials such as Ti.74 However, techniques such as through mask anodization have allowed reproducible features to be created.73,75,76 Such surfaces can be used to produce highly reproducible cell effects. These surfaces can reduce73 or increase cell spreading and MSC differentiation in vivo or in vitro.77–81 Such precise nanotopographical tools will help to dissect the rules of cell-topographical interactions and how they can be useful for work with Ti more simply than using, for example, roughness or random patterns.
Types of coating
There are three ways to change the physical, chemical and mechanical properties of surfaces: (1) by adding a new layer to the surface, (2) by changing the surface itself by exposure to physical or chemical agents like plasma or wet chemicals or (3) by subtraction or attrition process to modify the mechanical surface. However, to achieve the nanoscale modifications it should be able to reach all the topography device surface, change it to reach the commercial scale to be finally industrially integrated.2,82–84
In mechanical modification, the changes are required to improve the adhesion, bonding and bio-mineralization by increasing the surface area.85 Surface mechanical attrition treatment (SMAT) is a novel technique developed to provide a surface roughness at the nanoscale. This increase in the surface roughness leads to increases in the adhesion energy which have a positive reflection on cellular response.86,87 The cons behind this technique is the flexibility limitation in controlling the intracellular response beyond local adhesion energy.2 There are three ways for chemical modification for metal surfaces: (1) physiochemical adsorption, (2) molecule covalent binding and (3) peptide inclusion into a carrier material. However, different methods including anodization, oxidative, biochemical functionalization, acid/alkaline treatment, chemical vapour and sol-gel process can affect biologically active moieties onto the surface by controlling the relative densities or arrangement that in turn may have an effect on cell signalling.2,88–90 Physical modification mainly involves the physical spraying of coating or atomic rearrangement with ion implantation.91 The common techniques used to change the physical components of the substrate include plasma and vapour deposition, ion implantation, thermal oxidation and laser irradiation. Plasma is the fourth type of matter that highly excites the atoms, ions or radial species. The vacuum deposition is using vacuum condensation of a thin material to coat the substrate, while during the ion implantation, selected ions can be deposited on the material surface. Moreover, the involvement of temperature leads to alteration of the crystal structure of the Ti oxide layer which generates a superficial stress or changing in the previous surface nanostructure.2 Previous studies showed the effect of various nanosurface modifications on enhancing osteoblast activity, spreading, proliferation, differentiation and osteoconduction.91–97
There are three ways of coating: organic, inorganic and combination of both. Organic coating such as polymers, biomimetic and bioinspired films like a component of natural cell surroundings and inorganic components such as calcium phosphate (CaP), HA, titanium oxide (TiO2) and nitride coating.82 The combination coating is also divided into many types: by their mode of action, type of biological reagent incorporated with (e.g. antibiotics), type of coating (e.g. biodegradable polymers, hydrogel or bioceramic), coating deposit (layer-by-layer, vacuum deposit or electrophoresis) and the coating function. The antibacterial combination coating was reported and discussed in detail in previous study.98 Tobin,99 in his review, discussed the three types of combination device coating: (1) reduced infection either by controlling the kinetics release or coating with low potential to induce microbial resistance, (2) enhanced device integration or (3) reduced infection and enhanced integration. A number of reviews have been published on the combination of different coating on orthopaedic implants98–101 and dental implants.46,82
Moreover, implant coating must meet a number of significant challenging requirements to achieve a successful clinical implementation: for instance, a sufficient mechanical integrity, minimization of the local/systemic cytotoxicity and genotoxicity, sufficient amount of the pharmaceutical or biologic agent in the excipient coating matrix, optimization of diffusion kinetics that are not impeded by attachment of proteins to the implant, broad spectrum of antibiotics against biofilm formation without indication a bacterial resistance, and for the technical parts: the coating should be produced with a low coat, easy to manufacture, easy handling, long shelf life and ability to sterilize using conventional sterilization techniques without damaging the incorporated drug or biologic agent. However, there is no current coating system that fulfils all of these requirements.100
Nevertheless, due to the differences in cell variability related to the species (rat, mouse or human) and cell type (stem cell, osteoblast, etc.), the ability to assess a different kind of coating on the cell structure and function could be challenging. To address this, in the next section (Table 1), we provide selected examples of the impact of different types of coating (organic/non-organic, physical and chemical coating) on Ti surfaces in vivo or in vitro studies.
Table 1.
Impact of different coatings on Ti surfaces.
| Coating type | Cell type | Findings | Study |
|---|---|---|---|
| Ti surfaces coated with poly(ethyl acrylate) (PEA) fibronectin (FN) and a low dose of BMP7 | In vitro: hMSCs | The current coating showed an improvement in cell adhesion proliferation, differentiation and mineralization on the surfaces coated with PEA/BMP7 in comparison to those coated with BMP7 only | Al-Jarsha et al.102 |
| Graphene (G) coating onto a Ti6Al4V surface | In vivo: rabbit femoral condyle defect model | G bioactivity and electrical property (asymmetric nanostructures, rigidity and roughness of a G layer) stimulate the osteogenic differentiation of G-Ti6Al4V implant that improved the initial fixation strength and long-term osteointegration of the implant/bone interface | Li et al.103 |
| Polymeric bilayer on Ti, obtained by layering of poly(acrylic acid) (PAA), then chitosan (CS) and gallium (Ga) | In vitro: MG63 osteoblast-like cells | The presence of PAA/CS-Ga bilayer did not affect cell growth. Ga upregulates bone morphogenetic protein (BMP2), a marker of early osteoblastic differentiation | Bonifacio et al.104 |
| Chitosan coating on Ti | In vitro: MC3T3-E1 (pre-osteoblasts) and C2C12 myoblasts | Both of cell lines spread successfully on Ti, but only C2C12 cells adhered to chitosan | Gilabert-chirivella et al.105 |
| Hydroxyapatite (HA) coating by micro-arc oxidation (MAO) process on Ti | In vitro: Murine pre-osteoblasts (MC3T3-E1) In vivo: rat |
MAO provided a composite coating that promoted cell proliferation In rats, the MAO increased the bonding strength between the bone tissues and implant and increased thickness of the oxide layer |
Hao et al.106 |
| Calcium phosphate (CaP) coatings on the surface of Ti plates | In vitro: human osteoblast-like MG-63 cell line | At concentration 6.0 × 10−3 mol of Ca(NO3)2·4H2O, 3.6 × 10−3 mol (NH4)H2PO4 and 0.1 mol NaNO3 the cells had a spindle shape with thick pseudopodia which provided strong adherence to the rough and porous surfaces | Sun et al.107 |
| CaTiO3 screws were implanted with/without HA coating | In vivo study (rabbit) | The CaTiO3 screws showed a higher compatibility and osseointegration compared with the HA-coated screws | Wang et al.108 |
| 1. Resorbable blast media (RBM) surface treated by HA as a control 2. Calcium and magnesium ions were implanted using plasma immersion ion implantation and deposition (PIIID) |
In vitro: Human bone marrow mesenchymal stem cells (hBM-MSCs) | 1. PIIID technique changed the surfaces chemistry but not the surface topography 2. Ion implantation either Ca or Mg showed an increase in cell attachment. At concentration 6.0 × 10−3 mol of Ca(NO3)2·4H2O, 3.6 × 10−3 mol (NH4)H2PO4 and 0.1 mol NaNO3 the cells had a spindle shape with thick pseudopodia which provided strong adhesion to the rough and porous surfaces |
Won et al.109 |
| Ti surfaces activated with piranha solution (a mixture of 1 part 30% H2O2 solution) and coated with bone sialoprotein (BSP) via physisorption or covalent coupling via an aminosilane linker (APTES) | In vitro: Primary human osteoblasts (hOBs) | 1. No significant difference in cell adhesion on Ti surface coated with BSP via physisorption compared to that of untreated Ti, while BSP application via covalent coupling caused reduction in cell adhesion 2. Ti surfaces coated with higher concentration of BSP increased cell migration 3. ALP activity was reduced in the BSP-coated Ti at the early stages of culture 4. Increase in calcium deposition was noted after 21 days in BSP-coated Ti |
Baranowski et al.110 |
| Ti-based Küntscher nails (K-nails) Plates with modified nanostructured coated with HA in a rat model |
In vitro: human osteosarcoma cell line (Saos-2/An1) In vivo: rat |
Both surface modifications significantly improved cell proliferation and alkaline phosphatase (ALP) activity compared with control Ti plates | Sirin et al.111 |
| Graphene oxide–chitosan–HA (GO–CS–HA) particles deposited on Ti substrates | In vitro: human fibroblasts (MG63) Staphylococcus aureus |
1. GO–CS–HA coatings could improve hydrophilicity of the surfaces and provide effective corrosion protection of the Ti substrate 2. After short culture (5 days), the coating showed no significant cytotoxic effects on MG63 cells 3. This coating could reduce the Staphylococcus aureus adhesion |
Shi et al.112 |
| Sandblasted Ti discs were immobilized with FN peptide: 1. FN (FN-Ti) 2. GRGDSP (Gly-Arg-Gly-Asp-Ser-Pro) (GRGDSP-Ti) 3. PHSRN (Pro-His-Ser-Arg-Asn) (PHSRN-Ti) 4. GRGDSP/PHSRN (GRGDSP/PHSRN-Ti) |
In vitro: osteoblast-like cells (MC3T3-E1) | 1. FN or FN-derived peptides enhanced cell adhesion and cell proliferation 2. Peptide-modified Ti surfaces provided enhanced osteogenic differentiation 3. FN and GRGDSP/PHSRN coating improved osteo-related gene expression |
Pramono et al.113 |
| Ca-PO nanostructure synthesis on brush-type Ti-organic nanostructured surface | In vitro: osteoblasts cell line (MS3T3-E1) | Nano-Ti surface with brush-type Ti-organic nanostructures and Ca-PO groups inclusions provided higher osteoblast adhesion | Zemtsova et al.114 |
| Graphene oxide (GO)-coated titanium (GO-Ti) substrate compared to sodium titanate (Na-Ti) substrate | In vitro: human periodontal ligament stem cells (PDLSCs) | The proliferation rate, ALP activity and up-regulation of osteogenesis-related markers were higher on GO-Ti compared to Na-Ti | Zhou et al.115 |
| Polytetrafluorethylene (PTFE) and Ti nitride (TiN) coatings | In vitro: MG-63 osteoblasts |
Ti coated with PTFE showed a delay in cell attachment after 48 h in culture, but after 168 h the cells present had higher viability/proliferation levels, expressed more ALP and osteocalcin (OC), and osteoprotegerin (OPG)/nuclear factor-kappa-B ligand (RANKL) ratio compared to uncoated Ti surface. TiN coating showed no effect on gene expression | Fleischmann et al.116 |
| Nano-coated TiO and Ca-HA-coated Ti samples by drop casting with NAFION (sulphonated tetrafluoro-ethylene based fluoropolymer-copolymer) membrane | In vitro: hOS | TiO nanoparticles surfaces showed greater cell adhesion and cell spreading compared to Ca-HA Ti surfaces | Nayar and Chakraverty117 |
| Ti soaked in simulated body fluid (SBF) on different time points | In vitro: pre-osteoblast cells (MC3T3-E1) | Ti with nanotubular topography led to a significant increase in apatite-forming ability and enhanced pre-osteoblast MC3T3 cell | Wang et al.118 |
| Human placental laminin or synthetic peptides | In vivo: rats In vitro: osteoblast-like cells (HOS and MG-63) |
The synthetic peptide promotes bone formation without any detectable antigenic activity in rats. While, in vitro, it showed an enhancement in bone cell function | Yeo et al.119 |
| Graphene oxide (Go)-Ti. | In vitro: MC3T3-E1 | Go-Ti increased the ALP activity and OCN expression and improved cell differentiation | Zhao et al.120 |
| Poly(ethylene glycol) (PEG) functionalized single-walled carbon nanotubes (SWCNTs) grafted on Ti surfaces | In vitro: Human osteosarcoma (CAL-72) | SWCNTs grafted on Ti had no cytotoxicity effect on osteoblast cells | Pan et al.121 |
| Ti nanopores (20-30-50 nm) were prepared by anodization of Ti at 5, 10 and 20 V in a mixture of fluorhydric and acetic acid | In vitro: hMSCs In vivo: implantation in rat tibias |
1. Ti30 and Ti50 nanostructures increased early osteoblastic gene differentiation without osteogenic supplements present 2. Ti nanopores enhanced the bone apposition and bone bonding strength in vivo in correlation with in vitro results |
Lavenus et al.80 |
| Ti-6Al-4V disc surfaces were coated with FN | In vitro: MC3T3-E1 cells (expect high levels of osteoblast differentiation) | At the concentration 1 nmol/L of FN, MC3T3 attachment increased to six- to eightfold compared with uncoated surfaces and increased the osteoblast gene marker expression | Rapuano et al.122 and Rapuano and MacDonald123 |
| Ti implant surfaces modified by laser beam with/without HA | In vivo study (rabbit) | Laser irradiation on Ti surfaces may increase osseointegration | Sisti et al.124 |
| Acid-etched titanium (AET) and laser-sintered titanium (LST) | In vitro: human dental pulp (DPSCs) and human osteoblasts | LST drove good levels of osteoblast differentiation from DPSCs with production of bone morphogenetic proteins and growth factors | Mangano et al.125 |
| Ti nanopores (30-150-300 nm) were prepared by physical vapour deposition | In vitro: hMSCs | 1. The integrins expression, cell morphology and osteoblastic differentiation were affected by nanopores Ti structure 2. Ti30 had more branched cell morphology compared with other surfaces 3. Ti30 and Ti150 nanostructured showed more osteogenic differentiation, while the Ti300 had a limited effect |
Lavenus et al.81 |
| Ti coated with: 1. HA 2. Type I collagen 3. Arg–Gly–Asp (RGD)–containing peptides |
In vitro: Human osteoprogenitor (HOP) cell | Increased HOP cell adhesion was observed | Le Guillou-Buffello et al.126 |
| GRGDSP peptide derived from FN coated on to Ti surfaces | In vitro: MC3T3-E1 | Peptide-coated Ti surfaces showed an increase in osteoblast-related gene markers | Yamamichi et al.127 |
| HA coating on Ti | In vitro: osteoblast-like rat cells | Cellular attachment to HA surfaces was slightly higher than titanium surfaces. HA crystallinity had no influence over initial cell adhesion in treated or control surfaces | Chang et al.128 |
| Calcium ion (Ca2+)-implanted Ti | In vivo study (rat) | New bone formed on Ti treated with Ca ions compared to untreated, with increased osteospecific gene expression | Hanawa et al.129 |
| Polystyrene culture dishes were coated with a 300 Ǻ titanium layer via electron beam evaporation followed by coating with glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine (GFOGER) peptide | In vitro: hBM-MSCs In vivo: rat cortical bone implant |
1. GFOGER on Ti enhanced osteoblastic differentiation and mineral deposition in hBM-MSCs, which lead to improvement of the osteoblastic function compared to unmodified Ti 2. The current coating significantly improved in vivo peri-implant bone regeneration and osseointegration 3. GFOGER-modified implants significantly triggered osseointegration compared to surfaces modified with full-length type I collagen |
Reyes et al.130 |
Surface modification and bacteria
Ti implants are designed to last around 20–25 years but around 10% fail prematurely with the most common cause being bacterial infection in the first year of implantation.131 Bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae and Pseudomonas aeruginosa for orthopaedic implants and Prevotella intermedia, Porphyromonas gingivalis and Fusobacterium nucleatum for dental implants are understood to play a major role in tissue inflammation and subsequent bone recession through peri-implantitis, osteolysis and osteomyelitis leading to premature implant failure.132,133
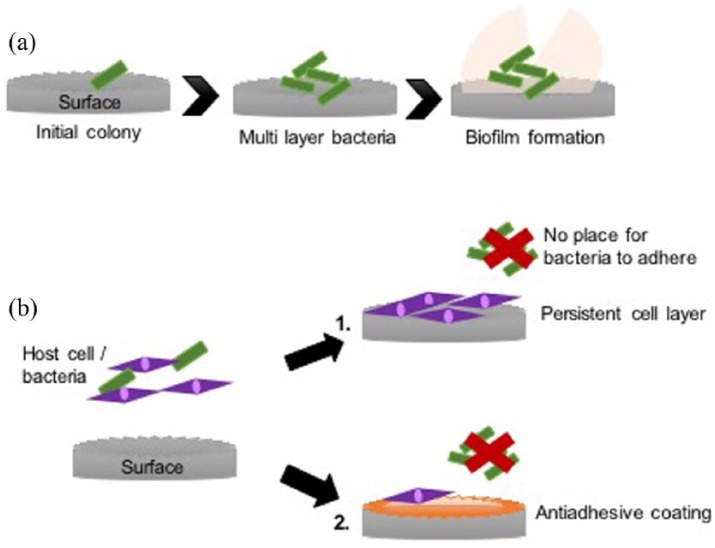
Many of these bacteria have the capability to form biofilms which can potentially occur within hours of initial bacteria attachment to an interface (Figure 2(a)). Primary colonizers such as Streptococci attach and proliferate to form microcolonies and secrete self-produced extracellular polymeric substances (EPS) such as proteins, extracellular DNA and exopolysaccharides to form a protective film or matrix. Once a surface has been populated, secondary colonizers, such as Porphyromonas gingivalis, are able to adhere to the sessile cells within the biofilm via receptors. Further aggregation, proliferation and EPS production result in a mature, multi-species biofilm containing a range of environments with varying nutrients and oxygen levels, allowing the bacteria to persist for long periods on the surface, causing chronic complications and becoming resistant to antibiotic treatment.134
Figure 2.
(a) The process of biofilm formation. Initially, cells attach, proliferate and coadhere to form microcolonies. They then continue to expand in similar fashion, together with production of EPS, to form a mature biofilm community. (b) Two possible ways to reduce implant infection: (1) provide no place for bacteria due to a continuous cell layer on the substrate and (2) use an antiadhesive coating that prevent bacterial attachment.
One of the superior qualities of Ti is its ability to absorb calcium, phosphate and serum proteins that are understood to accelerate and support osseointegration. However, such beneficial characteristics may also promote unfavourable processes such as bacterial adhesion.135
As implants have no resident microbiota to provide colonization resistance, they are susceptible to attachment by incoming microbes. Ideally, a surface should be designed to have selective activity against different cell types, mammalian cells or bacterial cells. Antiadhesive coatings have been created to repel bacteria from the surface and prevent attachment, thus inhibiting biofilms at the first stage. If this surface was then conversely encouraging host stem cells to adhere, proliferate, mature and differentiate, producing a continual cell layer before bacteria are able attach to the surface, implant infection and biofilm growth will be reduced or inhibited altogether (Figure 2(b)). However, it is important to note that the antiadhesive coating may reduce the mammalian cell attachment; hence, surface modifications combining antiadhesive polymers with cell adhesive motifs (e.g. FN, RGD) would be the ideal solution.102,113,126,127
There are two arguments about the effect of surface roughness on bacterial adhesion. The first scenario is that more bacteria adhere as surface microscale roughness increases due to the increased surface area that provides more binding sites and protection. The other argument is that increasing the surface roughness on the nanoscale may provide an unfavourable situation for the bacteria to adhere since the bacteria size is in microscale.
Regarding topography, however, after seminal reports showing that high aspect ratio topographies can kill bacteria, surfaces that can promote osteogenesis and prevent infection are being sought.136–143 The use of such high aspect features has been demonstrated in Ti in several new reports.142,143 In fact, it is becoming clear that both physical and chemical parameters play a role in potentially controlling bacterial adhesion (Table 2).
Table 2.
Examples of the effect of different coatings on bacterial adhesion.
| Ti treatment | Model bacteria | Findings | Study |
|---|---|---|---|
| A combination of silver, TiO2 and hydroxyapatite (HA) nanocoatings | S. sanguinis | A dual layer of silver-HA showed a significant reduction in biofilm formation compared with uncoated Ti or TiO2 nanocoatings | Besinis et al.144 |
| Polymeric bilayers on Ti, obtained by layers of poly(acrylic acid) (PAA), then Chitosan (CS) and Gallium (Ga) | E. coli, P. aeruginosa | The PAA-CS-Ga coatings released Ga(III) ions which has an antimicrobial effect | Bonifacio et al.104 |
| TiAl6V4 coated with multi-walled carbon nanotube (MWCNT) and impregnated with rifampicin antibiotic | S. epidermidis | CNTs are biologically compatible and can be utilized as drug delivery systems. MWCNT-modified surfaces showed a significant inhibition of biofilm formation up to 5 days culture | Hirschfeld et al.145 |
| Ti-O or Ti-I (iodine) | S. aureus | Ti surfaces coated with iodine showed a significant growth inhibition compared to Ti or Ti-O | Inoue et al.146 |
| Ti-copper oxide (TiCuO) coating | S. epidermidis | TiCuO can act as an antibacterial environment while remaining relatively nontoxic to a human osteoblast cell line | Norambuena et al.147 |
| Polyhydroxybutyrate (PHB) and its copolymer, polyhydroxybutyrate-co-hydroxyvalerate (PHBV) and gentamicin antibiotic | E. coli, S. aureus | PHBV coatings showed a faster degradation and more stable drug release (gentamicin) than PHB | Rodríguez-Contrerasa et al.148 |
| Ti surfaces coated with three layers: nanocrystalline HA, silver nanoparticles and calcium phosphate (either 150 or 1000 nm thick) | E. coli | An antimicrobial effect against E. coli was found with a 150 nm thick outer layer of the calcium phosphate | Surmeneva et al.149 |
| Polydopamine coating with silver nanoparticles on TiO2 nanotube arrays (Ag-PDA-TiO2) | E. coli | The antibacterial effect of Ag-PDA-TiO2 lasted longer than Ag-PDA-TiO or Ag-TiO2 (UV) effect | Xu et al.150 |
| Microgroove titanium functionalized with the AMP GL13K | P. gingivalis | Reduced the adhesion of bacteria over 72 h and promoted and adhesion and proliferation of human gingival fibroblasts | Zhou et al.151 |
| Titanium nanotubes coated with calcium phosphate and phospholipid impregnated with the AMP HHC-36 | S. aureus, P. aeruginosa | Able to kill bacteria and reduce adhesion to surface over 24 h | Kazemzadeh-Narbat et al.152 |
| Chimeric peptides functionalized onto titanium surfaces | S. oralis, S. gordonii, S. sanguinis | Functionalized surfaced showed antibacterial and anti-biofilm capabilities along with cyto-compatibility | Geng et al.153 |
| Deoxyribonuclease I (DNase I) | S. mutans, S. aureus | DNase I coating showed significant prevention of bacterial biofilms over a time of 24 h | Ye et al.154 |
| Ti surface coated with pure magnesium | S. epidermidis | Colony forming unit (CFU) counts decreased over time | Zaatreh et al.155 |
| Melamine (cationic peptide) | S. aureus, P. aeruginosa | Melamine treatment significantly inhibited biofilm formation by P. aeruginosa by up to 62% and S. aureus by up to 84% on the Ti substrates | Chen et al.156 |
| Cubic yttria-stabilized zirconia (YSZ) and Ag-YSZ nanocomposite films were deposited on Ti–6Al–V | S. aureus, S. epidermidis | The Ag-YSZ combination is a potential candidate for clinical application due to the broad-spectrum antimicrobial activity and low risk of resistance development to silver nanoparticles | Pérez-Tanoira et al.157 |
| Ti coated with covalent immobilized alkaline phosphates (ALP) on carboxymethyl chitosan (CMCS)-coated polydopamine (PDA) | S. epidermidis | This coating caused almost 89% reduction of the bacterial adhesion compared with uncoated surfaces | Zheng et al.158 |
| Ti coated with phosphatidylcholine mixed with amikacin or vancomycin or a combination of both | S. aureus, P. aeruginosa | Antibiotic-loaded coatings inhibited biofilm formation | Jennings et al.159 |
| Ti, zirconia and resin coated with saliva | S. sanguinis | Resin has a higher bacterial adhesion compared to Ti and zirconia | Lee et al.160 |
Anti-bacterial, high aspect ratio topographies, in fact, exist in nature. For example, cicada and dragonfly wings have topography that has been shown to be able to disrupt the bacterial membrane leading to cell lysis.136 Chemical and physical methods are now being developed to fabricate such topographies on clinically relevant materials like titanium, making the prospect of limiting implant infections while inside the body possible and reducing the rates of revision surgery and antibiotic treatment.
A titanium alloy, Ti-6Al-4V, has been developed using thermal oxidation to create a range of titanium dioxide nanostructures and through fluorescence studies, scanning electron microscope (SEM), transmission electron microscope (TEM) and focused ion beam scanning electron microscopy (FIB-SEM) has been shown to disrupt the bacterial membranes, ultimately leading to 40% E. coli cell death after 2-h incubation on the surface.161,162 Furthermore, previous studies showed that the TiO2 nanowires interact with the lipopolysaccharide and proteins, which are held together by electrostatic interactions with divalent cations. These interactions are essential to stabilize the outer membrane helping the TiO2 nanowires to form a molecular linkage at the cell surface allowing it to disturb bacteria membrane function which lead to the lysis of the bacteria. However, this is not the case in Gram-positive bacteria, where no antimicrobial activity has been observed as there might be no interaction of TiO2 nanowires with lipoteichoic acid that is present in the outer membrane of Gram-positive bacteria.163–165
Hydrothermal etching has been used to create topography in the micron range to produce a hierarchically ordered array shown to physically rupture S. aureus and P. aeruginosa cells leading to loss of viability seen in P. aeruginosa; 47.1% death compared to S. aureus with 19.8% death after 18 h of incubation.166 A chlorine base etching process has also been reported to form anisotropic nanostructures on the surface of titanium with a height of approximately 1 µm. The morphology of P. aeruginosa and S. aureus was significantly altered which correlated well with fluorescence studies showing high bactericidal activity for the Gram-negative bacteria with 98% ± 2% for P. aeruginosa and 95% ± 5% for E. coli after 4 h. For the Gram-positive bacteria S. aureus, there was less killing, with 22% ± 8% non-viable cells after 4 h, but this increased to 76% ± 4% after 24 h.139
Alkaline hydrothermal processes use sodium hydroxide, high temperatures and pressures to form titanium dioxide nano- and microscale topography on titanium substrates. Using electron microscopy, bacterial cell envelopes have been shown to be pierced by these spikes.161 Using fluorescence microscopy, loss of viability has been reported when in contact with this nanotopography.141,142,167 Diu et al.167 reported that motile bacteria (P. aeruginosa, E. coli and B. subtilis) were more liable to lysis with more than 50% cell death in the first hour while non-motile bacteria (S. aureus, E. faecalis and K. pneumoniae) experienced less than 5%. Not only has membrane disruption been seen but anti-biofilm activity has also been shown. Different structures of nanotopography have also been formed using alkaline hydrothermal method, a ‘spear-type’ topography and ‘pocket-type’ topography. After 6 days, there was half as much growth on the spear-type and five times less on the pocket-type compared to a control of flat-polished titanium.141 Tsimbouri et al.142 reported ~30% bacterial death after 1-h incubation of P. aeruginosa, which increased to 58% after 18 h incubation.
Along with having a bactericidal surface, it is important to ensure mammalian cells are able to attach, proliferate, mature and differentiate into desired lineages such as osteoblasts to promote successful osseointegration. Research suggests that the nanotopography is able to support osteoblast maturation through expression of osteogenic marker proteins such as Runt-related transcription factor (RUNX-2), BMP2, osteocalcin (OCN) and osteopontin (OPN).139,142,143,166,167
To improve osteointegration, various coatings have been utilized; for example, integrin-binding peptidic ligands have been functionalized onto nanotopographies and shown to significantly increase human mesenchymal stem cells (hMSCs) surface area and decrease the cell’s circularity evidence of improving surface interaction.143
Table 2 highlights various studies where titanium surfaces have been modified to reduce bacterial adhesion. Coatings with metals such as copper, gallium and silver with well-documented antimicrobial properties have shown to reduce biofilm formation of various bacteria.104,144,147,149–151 Antibiotics such as gentamicin are widely used in the treatment of both Gram-positive and Gram-negative bacteria and have been shown to have potential to be used as a coating on titanium surfaces.141 Antimicrobial peptides (AMPs) are recognized as promising candidates as alternatives for antibiotics due to the low chance of resistance being induced. Various AMPs have been functionalized on titanium such as HHC-36, GL13K and TBP-1 and have shown potential by reducing biofilm formation of both Gram-positive and Gram-negative bacteria.151–153
Conclusion
The main aim of bone implant industry is to mimic the normal function of tissue by enhancing the implant biocompatibility and reduce the bacterial adhesion while providing mechanical support. Recently, the coating of Ti implants has generated much interest in order to improve osseointegration and prevent unfavourable tissue reactions such as infection, inflammation and the foreign body response. Besides that, coated implants must be shown to be safe, efficient and cost-effective prior to subsequent adoption and widespread usage. Osseointegration/biofilm reduction are required goals; coating the implant with organic/inorganic components, changing the surface topography, and so on have been shown to be efficacious. In addition, the coating composition, location, thickness, uniformity and other physico-chemical variables are important to determine the efficacy and validity of the different coating.
This article aims to provide an overview of the impact of different physical and chemical modifications on Ti surface topography. Such alterations can potentially be used to enhance bone formation, provide bacterial growth inhibition or even perhaps both. That implant surface characteristics including surface roughness, surface chemistry, nanotopography to list a few, have a significant influence on osteogenesis and microbiota inhibition is emerging. Previous studies have implicated high aspect ratio nanofeatures with bactericidal potential. While an ideal implant would be both osseoinductive and antimicrobial, many aspects of these interactions require more investigation to resolve areas of uncertainty surrounding the interaction between these surfaces and MSCs when combined with bacteria. To conclude, the potential of Ti surface modifications is largely due to the ageing population placing pressure on orthopaedic treatments and Ti being the gold standard for fabrication on dental or orthopaedic implants.
Acknowledgments
We would like to acknowledge the mentorship and guidance of Professor Adam Curtis. Adam opened up the area of cell – topographical interactions to the world and we are grateful for his advice and friendship over the years. The authors thank Carol-Anne Smith for her technical support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a studentship to L.D. from University of Jeddah, Jeddah, Saudi Arabia and EPSRC grant EP/K034898/1.
ORCID iDs: Laila Damiati  https://orcid.org/0000-0002-4746-0915
https://orcid.org/0000-0002-4746-0915
Manuel Salmeron-Sanchez  https://orcid.org/0000-0002-8112-2100
https://orcid.org/0000-0002-8112-2100
References
- 1. Oldani C, Dominguez A. Titanium as a biomaterial for implants. In: Fokter S. (ed.) Recent advances in arthroplasty. Arthroplast InTech, 2012, pp. 149–162. [Google Scholar]
- 2. Staruch R, Griffin M, Butler P. Nanoscale surface modifications of orthopaedic implants: state of the art and perspectives. Open Orthop J 2016; 10: 920–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saini M, Singh Y, Arora P, et al. Implant biomaterials: a comprehensive review. World J Clin Cases 2015; 3(1): 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ananth H, Kundapur V, Mohammed HS, et al. A review on biomaterials in dental implantology. Int J Biomed Sci 2015; 11(3): 113–120. [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Yang C, Zhao H, et al. New developments of Ti-based alloys for biomedical applications. Materials 2014; 7(3): 1709–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Civantos A, Martínez-Campos E, Ramos V, et al. Titanium coatings and surface modifications: toward clinically useful bioactive implants. ACS Biomater Sci Eng 2017; 3(7): 1245–1261. [DOI] [PubMed] [Google Scholar]
- 7. Neoh KG, Hu X, Zheng D, et al. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012; 33(10): 2813–2822. [DOI] [PubMed] [Google Scholar]
- 8. De Jonge LT, Leeuwenburgh SCG, Wolke JGC, et al. Organic-inorganic surface modifications for titanium implant surfaces. Pharm Res 2008; 25(10): 2357–2369. [DOI] [PubMed] [Google Scholar]
- 9. Braceras I, Alava JI, Goikoetxea L, et al. Interaction of engineered surfaces with the living world: ion implantation vs. osseointegration. Surf Coat Tech 2007; 201(19–20): 8091–8098. [Google Scholar]
- 10. Asri RIM, Harun WSW, Samykano M, et al. Corrosion and surface modification on biocompatible metals: a review. Mater Sci Eng C 2017; 77: 1261–1274. [DOI] [PubMed] [Google Scholar]
- 11. Koh C, Atala A. Tissue engineering, stem cells, and cloning: opportunities for regenerative medicine. J Am Soc Nephrol 2004; 15(5): 1113–1125. [DOI] [PubMed] [Google Scholar]
- 12. Sansone V, Pagani D, Melato M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin Cases Miner Bone Metab 2013; 10(1): 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang JX, Fan YB, Gao Y, et al. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 2009; 30(27): 4590–4600. [DOI] [PubMed] [Google Scholar]
- 14. Daley B, Doherty AT, Fairman B, et al. Wear debris from hip or knee replacements causes chromosomal damage in human cells in tissue culture. J Bone Joint Surg Br 2004; 86(4): 598–606. [PubMed] [Google Scholar]
- 15. Egusa H, Ko N, Shimazu T, et al. Suspected association of an allergic reaction with titanium dental implants: a clinical report. J Prosthet Dent 2008; 100(5): 344–347. [DOI] [PubMed] [Google Scholar]
- 16. Lin L, Wang H, Ni M, et al. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J Orthop Transl 2014; 2(1): 35–42. [Google Scholar]
- 17. Bagno A, Di Bello C. Surface treatments and roughness properties of Ti-based biomaterials. J Mater Sci Mater Med 2004; 15(9): 935–949. [DOI] [PubMed] [Google Scholar]
- 18. Xiao J, Zhou H, Zhao L, et al. The effect of hierarchical micro/nanosurface titanium implant on osseointegration in ovariectomized sheep. Osteoporos Int 2011; 22(6): 1907–1913. [DOI] [PubMed] [Google Scholar]
- 19. Citeau A, Guicheux J, Vinatier C, et al. In vitro biological effects of titanium rough surface obtained by calcium phosphate grid blasting. Biomaterials 2005; 26(2): 157–165. [DOI] [PubMed] [Google Scholar]
- 20. Spatz JP, Geiger B. Molecular engineering of cellular environments: cell adhesion to nano-digital surfaces. Methods Cell Biol 2007; 83(1): 89–111. [DOI] [PubMed] [Google Scholar]
- 21. Anderson HJ, Sahoo JK, Ulijn RV, et al. Mesenchymal stem cell fate: applying biomaterials for control of stem cell behavior. Front Bioeng Biotechnol 2016; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6(4): 483–495. [DOI] [PubMed] [Google Scholar]
- 23. Kuroda S, Yamada K, Deguchi T, et al. Root proximity is a major factor for screw failure in orthodontic anchorage. Am J Orthod Dentofac Orthop 2007; 131(4 Suppl): 68–73. [DOI] [PubMed] [Google Scholar]
- 24. Shiu HT, Goss B, Lutton C, et al. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng Part B Rev 2014; 20(6): 697–712. [DOI] [PubMed] [Google Scholar]
- 25. Kasner E, Hunter CA, Ph D, et al. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater 2014; 10(8): 3363–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pier-Francesco A, Adams RJ, Waters MGJ, et al. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: an in vitro study. Clin Oral Implants Res 2006; 17(6): 633–637. [DOI] [PubMed] [Google Scholar]
- 27. Pye AD, Lockhart DEA, Dawson MP, et al. A review of dental implants and infection. J Hosp Infect 2009; 72(2): 104–110. [DOI] [PubMed] [Google Scholar]
- 28. Borba M, Deluiz D, Lourenço EJV, et al. Risk factors for implant failure: a retrospective study in an educational institution using GEE analyses. Braz Oral Res 2017; 31: e69. [DOI] [PubMed] [Google Scholar]
- 29. French D, Larjava H, Ofec R. Retrospective cohort study of 4591 Straumann implants in private practice setting, with up to 10-year follow-up. Part 1: multivariate survival analysis. Clin Oral Implants Res 2015; 26(11): 1345–1354. [DOI] [PubMed] [Google Scholar]
- 30. Becker ST, Beck-Broichsitter BE, Rossmann CM, et al. Long-term survival of Straumann dental implants with TPS surfaces: a retrospective study with a follow-up of 12 to 23 years. Clin Implant Dent Relat Res 2016; 18(3): 480–488. [DOI] [PubMed] [Google Scholar]
- 31. Heijdenrijk K, Raghoebar GM, Meijer HJ, et al. Two-part implants inserted in a one-stage or a two-stage procedure. A prospective comparative study. J Clin Periodontol 2002; 29(10): 901–909. [DOI] [PubMed] [Google Scholar]
- 32. Brady RA, Calhoun JH, Leid JG, et al. Infections of orthopaedic implants and device. In: Shirtliff M, Leid JG. (eds) The role of biofilms in device-related infections. Berlin: Springer, 2008, pp. 15–55. [Google Scholar]
- 33. Gottenbos B, Busscher HJ, Van Der Mei HC, et al. Pathogenesis and prevention of biomaterial centered infections. J Mater Sci Mater Med 2002; 13(8): 717–722. [DOI] [PubMed] [Google Scholar]
- 34. Rabih O, Darouiche MD, Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350(14): 1422–1429. [DOI] [PubMed] [Google Scholar]
- 35. Gray ED, Verstegen M, Peters G, et al. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet 1984; 323(8373): 365–367. [DOI] [PubMed] [Google Scholar]
- 36. Duguid IG, Evans E, Brown MRW, et al. Effect of biofilm culture upon the susceptibility of Staphylococcus epidermidis to tobramycin. J Antimicrob Chemother 1992; 30(6): 803–810. [DOI] [PubMed] [Google Scholar]
- 37. Albrektsson T, Wennerberg A. Oral implant surfaces: part 1 – review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 2004; 17(5): 536–543. [PubMed] [Google Scholar]
- 38. Hench LL. The story of Bioglass®. J Mater Sci Mater Med 2006; 17(11): 967–978. [DOI] [PubMed] [Google Scholar]
- 39. Hanson ET, Lewis RL, Auerbach R, et al. Third-generation biomedical materials. Science 2002; 295: 1014–1017. [DOI] [PubMed] [Google Scholar]
- 40. Bostman O, Hirvensalo E, Makinen J. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Joint Surg Br 1990; 72(4): 592–596. [DOI] [PubMed] [Google Scholar]
- 41. Henkel J, Woodruff MA, Epari DR, et al. Bone regeneration based on tissue engineering conceptions – a 21st century perspective. Bone Res 2013; 1(3): 216–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choi C, Kim M. Wettability effects on heat transfer. In: Ahsan A. (ed.) Two phase flow, phase change and numerical modeling. Intechopen, 2010, pp. 311–341. [Google Scholar]
- 43. Shafrin EG, Zisman WA. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers. J Phys Chem 1960; 64(5): 519–524. [Google Scholar]
- 44. Yuan Y, Lee TR. Contact angle and wetting properties. In: Bracco G, Holst B. (eds) Surface science techniques. Berlin: Springer, 2013, pp. 3–34. [Google Scholar]
- 45. De Gennes PG. Wetting: statics and dynamics. Rev Mod Phys 1985; 57(3): 827–863. [Google Scholar]
- 46. Le Guéhennec L, Soueidan A, Layrolle P, et al. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater 2007; 23(7): 844–854. [DOI] [PubMed] [Google Scholar]
- 47. Mendonça G, Mendonça DBS, Aragão FJL, et al. Advancing dental implant surface technology – from micron- to nanotopography. Biomaterials 2008; 29(28): 3822–3835. [DOI] [PubMed] [Google Scholar]
- 48. Sasaki K, Osamu S, Takahashi N. Surface modification of dental implant improves implant–tissue interface. In: Sasaki K, Suzuki O, Takahashi N. (eds) Interface oral health science. Tokyo: Springer, 2015, pp. 33–44. [Google Scholar]
- 49. Boyan BD, Hummert TW, Dean DD, et al. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996; 17(2): 137–146. [DOI] [PubMed] [Google Scholar]
- 50. Berglundh T, Abrahamsson I, Albouy JP, et al. Bone healing at implants with a fluoride-modified surface: an experimental study in dogs. Clin Oral Implants Res 2007; 18(2): 147–152. [DOI] [PubMed] [Google Scholar]
- 51. Isa ZM, Schneider GB, Zaharias R, et al. Effects of fluoride-modified titanium surfaces on osteoblast proliferation and gene expression. Int J Oral Maxillofac Implants 2005; 21(2): 203–211. [PubMed] [Google Scholar]
- 52. Dunn DS, Raghavan S, Volz RG. Anodized layers on titanium and titanium alloy orthopedic materials for antimicrobial activity applications. Mater Manuf Process 1992; 7(1): 123–137. [Google Scholar]
- 53. Shibata Y, Kawai H, Yamamoto H, et al. Antibacterial titanium plate anodized by being discharged in NaCl solution exhibits cell compatibility. J Dent Res 2004; 83: 115–119. [DOI] [PubMed] [Google Scholar]
- 54. Tejero R, Anitua E, Orive G. Toward the biomimetic implant surface: biopolymers on titanium-based implants for bone regeneration. Prog Polym Sci 2014; 39(7): 1406–1447. [Google Scholar]
- 55. Morra M, Cassinelli C, Cascardo G, et al. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials 2003; 24(25): 4639–4654. [DOI] [PubMed] [Google Scholar]
- 56. Cao H, Liu X. Activating titanium oxide coatings for orthopedic implants. Surf Coat Technol 2013; 233: 57–64. [Google Scholar]
- 57. Silvero C, Rocca MJ, de la Villarmois DM, et al. Selective photoinduced antibacterial activity of amoxicillin-coated gold nanoparticles: from one-step synthesis to in vivo cytocompatibility. ACS Omega 2018; 3(1): 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sul YT, Johansson CB, Kang Y, et al. Bone reactions to oxidized titanium implants with electrochemical anion sulphuric acid and phosphoric acid incorporation. Clin Implant Dent Relat Res 2002; 4(2): 78–87. [DOI] [PubMed] [Google Scholar]
- 59. Krishna Alla R, Ginjupalli K, Upadhya N, et al. Surface roughness of implants: a review. Trends Biomater Artif Organs 2011; 25(3): 112–118. [Google Scholar]
- 60. Mehta R, Panda S, Nanda S, et al. Implant surface modification and osseointegration – past, present and future. J Oral Heal Community Dent 2014; 8: 113–118. [Google Scholar]
- 61. Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants 2009; 25(1): 63–74. [PubMed] [Google Scholar]
- 62. Wennerberg A, Albrektsson T, Wennerberg AAT. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants 2000; 15(3): 331–344. [PubMed] [Google Scholar]
- 63. Sahib A, Al-radha D, Dymock D, et al. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J Dent 2011; 40: 146–153. [DOI] [PubMed] [Google Scholar]
- 64. Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. J Clin Periodontal 1995; 22: 1–14. [DOI] [PubMed] [Google Scholar]
- 65. Bolle C, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater 1997; 13: 258–269. [DOI] [PubMed] [Google Scholar]
- 66. Larsson C, Thomsen P, Lausmaa J, et al. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials 1994; 15(13): 1062–1074. [DOI] [PubMed] [Google Scholar]
- 67. Yang G, He F, Yang X. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: 516–524. [DOI] [PubMed] [Google Scholar]
- 68. Nishimura I, Huang Y, Butz F, et al. Discrete deposition of hydroxyapatite nanoparticles on a titanium implant with predisposing substrate microtopography accelerated osseointegration. Nanotechnology 2007; 18: 245101. [Google Scholar]
- 69. Oliveira PT, De Zalzal SF, Beloti MM, et al. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A 2006; 80A: 554–564. [DOI] [PubMed] [Google Scholar]
- 70. Carbone R, Marangi I, Zanardi A, et al. Biocompatibility of cluster-assembled nanostructured TiO2 with primary and cancer cells. Biomaterials 2006; 27: 3221–3229. [DOI] [PubMed] [Google Scholar]
- 71. Kommireddy DS, Sriram SM, Lvov YM, et al. Stem cell attachment to layer-by-layer assembled TiO2 nanoparticle thin films. Biomaterials 2006; 27: 4296–4303. [DOI] [PubMed] [Google Scholar]
- 72. Huang H, Pan S, Lai Y, et al. Osteoblast-like cell initial adhesion onto a network-structured titanium oxide layer 2004; 51: 1017–1021. [Google Scholar]
- 73. Sjo T, Dalby MJ, Hart A, et al. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater 2009; 5: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 74. Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater 2014; 13(6): 558–569. [DOI] [PubMed] [Google Scholar]
- 75. Sjöström T, Mcnamara LE, Meek RMD, et al. 2D and 3D nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Adv Healthc Mater 2013; 2: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 76. Sjöström T, Fox N, Su B. Through-mask anodization of titania dot- and pillar-like nanostructures on bulk Ti substrates using a nanoporous anodic alumina mask. Nanotechnology 2009; 20(13): 135305. [DOI] [PubMed] [Google Scholar]
- 77. Mcnamara LE, Sjöström T, Seunarine K, et al. Investigation of the limits of nanoscale filopodial interactions. J Tissue Eng. Epub ahead of print 13 May 2014. DOI: 10.1177/2041731414536177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mcnamara LE, Sjöström T, Burgess KEV, et al. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials 2011; 32: 7403–7410. [DOI] [PubMed] [Google Scholar]
- 79. Dalby MJ, García AJ, Salmeron-Sanchez M. Receptor control in mesenchymal stem cell engineering. Nat Rev Mater 2018; 3: 17091. [Google Scholar]
- 80. Lavenus S, Trichet V, Le Chevalier S, et al. Cell differentiation and osseointegration influenced by nanoscale anodized titanium surfaces. Nanomedicine 2012; 7(7): 967–980. [DOI] [PubMed] [Google Scholar]
- 81. Lavenus S, Berreur M, Trichet V, et al. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur Cell Mater 2011; 22: 84–96. [DOI] [PubMed] [Google Scholar]
- 82. Mandracci P, Mussano F, Rivolo P, et al. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016; 6(1): 7. [Google Scholar]
- 83. Varíola F, Vetrone F, Richert L, et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: an overview of strategies, fabrication methods, and challenges. Small 2009; 5(9): 996–1006. [DOI] [PubMed] [Google Scholar]
- 84. Variola F, Yi JH, Richert L, et al. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008; 29(10): 1285–1298. [DOI] [PubMed] [Google Scholar]
- 85. Lausmaa J. Mechanical, thermal, chemical and electrochemical surface treatment of titanium. In: Titanium in medicine: material science, surface science, engineering, biological responses and medical applications. Berlin; Heidelberg: Springer, 2001. pp. 231–266, 10.1007/978-3-642-56486-4_8 [DOI] [Google Scholar]
- 86. Lu K, Lu J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mater Sci Eng A 2004; 375–377(1–2): 38–45. [Google Scholar]
- 87. Zhang HW, Hei ZK, Liu G, et al. Formation of nanostructured surface layer on AISI 304 stainless steel by means of surface mechanical attrition treatment. Acta Mater 2003; 51(7): 1871–1881. [Google Scholar]
- 88. Ginsberg MH, Rd P. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci 1991; 16: 246–250. [DOI] [PubMed] [Google Scholar]
- 89. Mark K, Von Der Park J, Bauer S. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res 2010; 339: 131–153. [DOI] [PubMed] [Google Scholar]
- 90. Thoneick M, Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res 2009; 20: 185–206. [DOI] [PubMed] [Google Scholar]
- 91. Reising A, Yao C, Storey D, et al. Greater osteoblast long-term functions on ionic plasma deposited nanostructured orthopedic implant coatings. J Biomed Mater Res A 2007; 87: 78–83. [DOI] [PubMed] [Google Scholar]
- 92. Ogawa T, Saruwatari L, Takeuchi K, et al. Ti nano-nodular structuring for bone integration and regeneration. J Dent Res 2008; 87: 751–756. [DOI] [PubMed] [Google Scholar]
- 93. Hovgaard MB, Rechendorff K, Chevallier J, et al. Fibronectin adsorption on tantalum: the influence of nanoroughness. J Phys Chem B 2008; 112: 8241–8249. [DOI] [PubMed] [Google Scholar]
- 94. Herzog M, Du A, Hannig M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implants Res 2006; 17(6): 736–745. [DOI] [PubMed] [Google Scholar]
- 95. Puckett SD, Taylor E, Raimondo T, et al. Biomaterials the relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010; 31(4): 706–713. [DOI] [PubMed] [Google Scholar]
- 96. Munirathinam B, Neelakantan L. Titania nanotubes from weak organic acid electrolyte: fabrication, characterization and oxide film properties. Mater Sci Eng C 2015; 49: 567–578. [DOI] [PubMed] [Google Scholar]
- 97. Mariscal-muñoz E, Costa CAS, Tavares HS, et al. Osteoblast differentiation is enhanced by a nano-to-micro hybrid titanium surface created by Yb: YAG laser irradiation. Clin Oral Investig 2016; 20: 503–511. [DOI] [PubMed] [Google Scholar]
- 98. Romanò CL, Scarponi S, Gallazzi E, et al. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res 2015; 10(1): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tobin EJ. Recent coating developments for combination devices in orthopedic and dental applications: a literature review. Adv Drug Deliv Rev 2017; 112: 88–100. [DOI] [PubMed] [Google Scholar]
- 100. Goodman SB, Yao Z, Keeney M, et al. The future of biologic coatings for orthopaedic implants. Biomaterials 2013; 34(13): 3174–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang BGX, Myers DE, Wallace GG, et al. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci 2014; 15(7): 11878–11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Al-Jarsha M, Moulisová V, Leal-Egaña A, et al. Engineered coatings for titanium implants to present ultralow doses of BMP-7. ACS Biomater Sci Eng 2018; 4(5): 1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li K, Wang C, Yan J, et al. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: an in vivo study. Sci Rep 2018; 8(1): 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bonifacio MA, Cometa S, Dicarlo M, et al. Gallium-modified chitosan/poly(acrylic acid) bilayer coatings for improved titanium implant performances. Carbohydr Polym 2017; 166: 348–357. [DOI] [PubMed] [Google Scholar]
- 105. Gilabert-chirivella E, Pérez-feito R, Ribeiro C, et al. Chitosan patterning on titanium implants. Prog Organ Coat 2017; 111: 23–28. [Google Scholar]
- 106. Hao J, Li Y, Wang X, et al. Corrosion resistance and biological properties of a micro–nano structured Ti surface consisting of TiO2 and hydroxyapatite. RSC Adv 2017; 7(53): 33285–33292. [Google Scholar]
- 107. Sun Q, Yang Y, Luo W, et al. The influence of electrolytic concentration on the electrochemical deposition of calcium phosphate coating on a direct laser metal forming surface. Int J Anal Chem 2017; 2017: 8610858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang Z, He R, Tu B, et al. Enhanced biocompatibility and osseointegration of calcium titanate coating on titanium screws in rabbit femur. J Huazhong Univ Sci Technol Med Sci 2017; 37: 362–370. [DOI] [PubMed] [Google Scholar]
- 109. Won S, Huh Y-H, Cho L-R, et al. Cellular response of human bone marrow derived mesenchymal stem cells to titanium surfaces implanted with calcium and magnesium ions. Tissue Eng Regen Med 2017; 14(2): 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Baranowski A, Klein A, Ritz U, et al. Surface functionalization of orthopedic titanium implants with bone sialoprotein. PLoS ONE 2016; 11(4): e0153978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sirin HT, Vargel I, Kutsal T, et al. Ti implants with nanostructured and HA-coated surfaces for improved osseointegration. Artif Cells Nanomedicine Biotechnol 2016; 44(3): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 112. Shi YY, Li M, Liu Q, et al. Electrophoretic deposition of graphene oxide reinforced chitosan-hydroxyapatite nanocomposite coatings on Ti substrate. J Mater Sci Mater Med 2016; 27: 48. [DOI] [PubMed] [Google Scholar]
- 113. Pramono S, Pugdee K, Suwanprateep J, et al. Sandblasting and fibronectin-derived peptide immobilization on titanium surface increase adhesion and differentiation of osteoblast-like cells (MC3T3-E1). J Dent Sci 2016; 11(4): 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zemtsova EG, Morozov PE, Valiev RZ, et al. The synthesis of titanium-organic nanostructures on nanotitanium surface for biocompatible coating development. Rev Adv Mater Sci 2016; 45(1–2): 59–66. [Google Scholar]
- 115. Zhou Q, Yang P, Li X, et al. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci Rep 2016; 6(1): 19343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fleischmann L, Crismani A, Falkensammer F, et al. Behavior of osteoblasts on Ti surface with two different coating designed for orthodontic devices. J Mater Sci Mater Med 2015; 26(1): 5335. [DOI] [PubMed] [Google Scholar]
- 117. Nayar S, Chakraverty S. A comparative study to evaluate the osteoblastic cell behavior of two nano coated titanium surfaces with NAFION stabilized the membrane. J Indian Prosthodont Soc 2015; 15(1): 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang H, Lai YK, Zheng RY, et al. Tuning the surface microstructure of titanate coatings on titanium implants for enhancing bioactivity of implants. Int J Nanomedicine 2015; 10: 3887–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yeo IS, Min SK, Ki Kang H, et al. Adhesion and spreading of osteoblast-like cells on surfaces coated with laminin-derived bioactive core peptides. Data Br 2015; 5: 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhao C, Lu X, Zanden C, et al. The promising application of graphene oxide as coating materials in orthopedic implants: preparation, characterization and cell behavior. Biomed Mater 2015; 10(1): 15019. [DOI] [PubMed] [Google Scholar]
- 121. Pan C-J, Dong Y-XD, Jandt K. Grafting carbon nanotubes on titanium surface for osteoblast cell adhesion and growth. J Biomater Nanobiotechnol 2012; 3(3): 353–361. [Google Scholar]
- 122. Rapuano B, Hackshaw K, Schniepp H, et al. Effects of coating a titanium alloy with fibronectin on the expression of osteoblast gene markers in the MC3T3 osteoprogenitor cell line. Int J Oral Maxillofac Implant 2012; 27(5): 1081–1090. [PMC free article] [PubMed] [Google Scholar]
- 123. Rapuano BE, MacDonald DE. Surface oxide net charge of a titanium alloy: modulation of fibronectin-activated attachment and spreading of osteogenic cells. Colloids Surf B Biointerfaces 2011; 82(1): 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sisti K, de Rossi R, Antoniolli-Brochado A, et al. Surface and biomechanical study of titanium implants modified by laser with and without hydroxyapatite coating, in rabbits. J Oral Implantol 2012; 38(3): 231–237. [DOI] [PubMed] [Google Scholar]
- 125. Mangano C, De Rosa A, Desiderio V, et al. The osteoblastic differentiation of dental pulp stem cells and bone formation on different titanium surface textures. Biomaterials 2010; 31(13): 3543–3551. [DOI] [PubMed] [Google Scholar]
- 126. Le Guillou-Buffello D, Bareille R, Gindre M, et al. Additive effect of RGD coating to functionalized titanium surfaces on human osteoprogenitor cell adhesion and spreading. Tissue Eng Part A 2008; 14(8): 1445–1455. [DOI] [PubMed] [Google Scholar]
- 127. Yamamichi N, Pugdee K, Chang W-J, et al. Gene expression monitoring in osteoblasts on titanium coated with fibronectin-derived peptide. Dent Mater J 2008; 27(5): 744–750. [DOI] [PubMed] [Google Scholar]
- 128. Chang YL, Stanford CM, Wefel JS, et al. Osteoblastic cell attachment to hydroxyapatite-coated implant surfaces in vitro. Int J Oral Maxillofac Implants 1999; 14(2): 239–247. [PubMed] [Google Scholar]
- 129. Hanawa T, Kamiura Y, Yamamoto S, et al. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J Biomed Mater Res A 1997; 361: 131–136. [DOI] [PubMed] [Google Scholar]
- 130. Reyes CD, Petrie T, Burns KL, et al. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2008; 28(21): 3228–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tripathy A, Sen P, Su B, et al. Natural and bioinspired nanostructured bactericidal surfaces. Adv Colloid Interface Sci 2017; 248: 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012; 2(4): 176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Holmberg KV, Abdolhosseini M, Li Y, et al. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater 2013; 9(9): 8224–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rickard AH, Gilbert P, High NJ, et al. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 2003; 11(2): 94–100. [DOI] [PubMed] [Google Scholar]
- 135. Yoshinari M, Oda Y, Kato T, et al. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 2001; 22(14): 2043–2048. [DOI] [PubMed] [Google Scholar]
- 136. Ivanova EP, Hasan J, Webb HK, et al. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012; 8(16): 2489–2494. [DOI] [PubMed] [Google Scholar]
- 137. Ivanova EP, Hasan J, Webb HK, et al. Bactericidal activity of black silicon. Nat Commun 2013; 4: 2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hasan J, Webb HK, Truong VK, et al. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl Microbiol Biotechnol 2013; 97: 9257–9262. [DOI] [PubMed] [Google Scholar]
- 139. Hasan J, Jain S, Chatterjee K. Nanoscale topography on black titanium imparts multi-biofunctional properties for orthopedic applications. Sci Rep 2017; 7: 41118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ostrikov K, Macgregor -M, Cavallaro A, et al. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology 2017; 28: 245301. [DOI] [PubMed] [Google Scholar]
- 141. Cao Y, Su B, Chinnaraj S, et al. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci Rep 2018; 8: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tsimbouri PM, Holloway N, Fisher L, et al. Osteogenic and bactericidal surfaces from hydrothermal titania nanowires on titanium substrates. Sci Rep 2016; 6: 36857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fraioli R, Tsimbouri PM, Fisher LE, et al. Towards the cell-instructive bactericidal substrate: exploring the combination of nanotopographical features and integrin selective synthetic ligands. Sci Rep 2017; 7: 16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Besinis A, Hadi SD, Le HR, et al. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017; 11(3): 327–338. [DOI] [PubMed] [Google Scholar]
- 145. Hirschfeld J, Akinoglu EM, Wirtz DC, et al. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine 2017; 13(4): 1587–1593. [DOI] [PubMed] [Google Scholar]
- 146. Inoue D, Kabata T, Ohtani K, et al. Inhibition of biofilm formation on iodine-supported titanium implants. Int Orthop 2017; 41(6): 1093–1099. [DOI] [PubMed] [Google Scholar]
- 147. Norambuena GA, Patel R, Karau M, et al. Antibacterial and biocompatible titanium-copper oxide coating may be a potential strategy to reduce periprosthetic infection: an in vitro study. Clin Orthop Relat Res 2017; 475(3): 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rodríguez-Contrerasa A, García Y, Manero JM, et al. Antibacterial PHAs coating for titanium implants. Eur Polym J 2017; 90: 66–78. [Google Scholar]
- 149. Surmeneva MA, Sharonova AA, Chernousova S, et al. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surf B Biointerfaces 2017; 156: 104–113. [DOI] [PubMed] [Google Scholar]
- 150. Xu J, Xu N, Zhou T, et al. Polydopamine coatings embedded with silver nanoparticles on nanostructured titania for long-lasting antibacterial effect. Surf Coat Technol 2017; 320: 608–613. [Google Scholar]
- 151. Zhou L, Lai Y, Huang W, et al. Biofunctionalization of microgroove titanium surfaces with an antimicrobial peptide to enhance their bactericidal activity and cytocompatibility. Colloids Surf B Biointerfaces 2015; 128: 552–560. [DOI] [PubMed] [Google Scholar]
- 152. Kazemzadeh-Narbat M, Lai BFL, Ding C, et al. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013; 34(24): 5969–5977. [DOI] [PubMed] [Google Scholar]
- 153. Geng H, Yuan Y, Adayi A, et al. Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants. Mater Sci Eng C 2018; 82: 141–154. [DOI] [PubMed] [Google Scholar]
- 154. Ye J, Shao C, Zhang X, et al. Effects of DNase I coating of titanium on bacteria adhesion and biofilm formation. Mater Sci Eng C 2017; 78: 738–747. [DOI] [PubMed] [Google Scholar]
- 155. Zaatreh S, Haffner D, Strauss M, et al. Thin magnesium layer confirmed as an antibacterial and biocompatible implant coating in a co-culture model. Mol Med Rep 2017; 15(4): 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chen R, Willcox MDP, Ho KKK, et al. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016; 85: 142–151. [DOI] [PubMed] [Google Scholar]
- 157. Pérez-Tanoira R, Horwat D, Kinnari TJ, et al. Bacterial adhesion on biomedical surfaces covered by yttria stabilized zirconia. J Mater Sci Mater Med 2016; 27(1): 6. [DOI] [PubMed] [Google Scholar]
- 158. Zheng D, Neoh KG, Kang ET. Bifunctional coating based on carboxymethyl chitosan with stable conjugated alkaline phosphatase for inhibiting bacterial adhesion and promoting osteogenic differentiation on titanium. Appl Surf Sci 2016; 360: 86–97. [Google Scholar]
- 159. Jennings JA, Carpenter DP, Troxel KS, et al. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin Orthop Relat Res 2015; 473(7): 2270–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lee B-C, Jung G-Y, Kim D-J, et al. Initial bacterial adhesion on resin, titanium and zirconia in vitro. J Adv Prosthodont 2011; 3(2): 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jenkins J, Nobbs AH, Verkade P, et al. Characterisation of bactericidal titanium surfaces using electron microscopy characterisation of bactericidal titanium surfaces using electron microscopy. Microsc Anal 2018; 34: 17–22. [Google Scholar]
- 162. Sjöström T, Nobbs AH, Su B. Bactericidal nanospike surfaces via thermal oxidation of Ti alloy substrates. Mater Lett 2016; 167: 22–26. [Google Scholar]
- 163. Visai L, De Nardo L, Punta C, et al. Titanium oxide antibacterial surfaces in biomedical devices. Int J Artif Organs 2011; 34(9): 929–946. [DOI] [PubMed] [Google Scholar]
- 164. Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002; 23(16): 3359–3368. [DOI] [PubMed] [Google Scholar]
- 165. Munisparan T, Yang ECY, Paramasivam R, et al. Optimisation of preparation conditions for Ti nanowires and suitability as an antibacterial material. IET Nanobiotechnol 2018; 12(4): 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bhadra CM, Khanh Truong V, Pham VTH, et al. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci Rep 2015; 5: 16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Diu T, Faruqui N, Sjöström T, et al. Cicada-inspired cell-instructive nanopatterned arrays. Sci Rep 2014; 4: 7122. [DOI] [PMC free article] [PubMed] [Google Scholar]