Abstract
Ionizing radiation (IR) can result in serious genomic instability and genotoxicity by causing DNA damage. Carbon ion (CI) beams and X-rays are typical IRs and possess high-linear energy transfer (LET) and low-LET, respectively. In this article, a comet assay that was optimized by decreasing the electrophoresis time (8 minutes) and voltage (0.5 V/cm) was performed to elucidate and quantify the DNA damage induced by CI or X-rays radiation. Two quantitative methods for the comet assay, namely, comet score and olive tail moment, were compared, and the appropriate means and parameter values were selected for the present assay. The dose–effect relationship for CI or X-rays radiation and the DNA repair process were studied in yeast cells. These results showed that the quadratic function fitted the dose–effect relationship after CI or X-rays exposure, and the trend for the models fitted the dose–effect curves for various repair times was precisely described by the cubic function. A kinetics model was also creatively used to describe the process of DNA repair, and equations were calculated within repairable ranges that could be used to roughly evaluate the process and time necessary for DNA repair.
Keywords: DNA damage, comet assay, DNA repair, model fitting, Saccharomyces cerevisiae
Introduction
Carbon ion (CI) beams and X-rays are representative models of high-linear energy transfer (LET) and low-LET ionizing radiation (IR), respectively. Ionizing radiation induces DNA single- and double-strand breaks (SSBs and DSBs) as well as several types of base damage in organisms.1–3 Single-strand breaks and base modifications can be efficiently repaired with high fidelity, mainly via base excision repair. The potent mutagenicity of CI is in part due to a greater incidence of clustered DSB formed when both strands of the phosphodiester DNA backbone are broken, and these clustered DSBs are responsible for the majority of lethal effects4 and genomic instability.5 The repair of such DNA damage occurs with slow kinetics and is highly reliant on the homologous recombination and nonhomologous end-joining pathways.6–9
The comet assay (single-cell gel electrophoresis) is a versatile, relatively simple, and sensitive measurement method that is already widely applied to study numerous aspects of DNA damage. It has multitudinous advantages; for example, only approximately a thousand cells are required for this assay, and the cells do not need to be tagged with a radioisotope, allowing the measurement of DNA damage in any nucleated cell. Perhaps most importantly, this assay is used to assess variations at the level of single cells in response to DNA damaging agents.10,11 Multiform DNA damage, for instance SSB, DSB, DNA cross-links, base damage, and apoptotic nuclei, and corresponding repair can be detected. The comet assay in alkaline conditions can be used to simultaneously detect combinations of DNA damage involving SSBs, DSBs, and alkali-labile sites.12–14
The DNA damage induced by IR shows a dose–response relationship, that is, a relationship between the dose and the degree of DNA damage, based on in vitro experiments.15,16 The detection limits, linearity of calibration curves, and repair rate of DNA damage induced by X-rays have been evaluated in multiple organs of the mouse by the comet assay.17 In addition, the comet assay is frequently applied to study radio protectants against IR-induced DNA lesions18 and pollution effects in the aquatic environment.19 Despite the range of applications of the comet assay in yeast, protozoa, plant, and invertebrate, the yeast comet assay has not been extensively applied because of the operative difficulties and analytical complications.20 Moreover, the relationship, and especially the function models, between doses of CI or X-rays radiation, and the effect of DNA damage and repair are still poorly described and require further investigation.
In this study, by means of combining preexisting protocols fitted for yeast cells21–23 and optimizing the method, the alkaline comet assay was used to examine the dose–effect relationship for yeast cell DNA damage after exposure to CI or X-rays, and the relationships and functional models of DNA repair in the system were described.
Materials and Methods
Strain, Medium, and Growth Conditions
The Saccharomyces cerevisiae strain used in the experiments was CICC 1308 (MATa, budding, haploid; obtained from the Center of Industrial Culture Collection of China). The preinoculum was diluted in fresh yeast peptone dextrose (YPD) medium (1% yeast extract, 2% peptone, and 2% glucose) to optical density (OD600) = 0.1. The final volume was 25 mL in a 100-mL Erlenmeyer flask, and it was incubated for approximately 9 hours (log phase) at 30°C and 200 rpm. Cells at log phase were used for irradiation and for comet analysis.
X-Rays or CI Beam Radiation
The aforementioned yeast cells were put into an individual sterilized dish of φ35 mm and divided into 8 groups at random. These samples were irradiated with X-rays or CI at doses of 0, 25, 50, 75, 100, 125, 150, and 175 Gy, successively. The radiations were conducted with the RX-650 X-ray biological irradiator (FAXITRON, Tucson, Arizona) and Heavy Ion Research Facility in Lanzhou at Institute of Modern Physics, Chinese Academy of Sciences. The energy, LET, and dose rates of CI beams were 100 MeV/u, 202 keV/μm, and 40 Gy/min, respectively. With regard to X-rays, the energy and dose rate were 100kVp and 1.5Gy/min, respectively. Control samples were handled in the same way, except for the irradiation treatment. In order to assess the process of DNA repair, the samples were preserved at −80°C after incubation in normal growth conditions (30°C and 200 rpm) for 3, 6, 12, and 24 hours.
Estimation of the Viable Population by Plate Count Method
After irradiation, the yeast cells were diluted 10-fold by sterilized water to the appropriate concentrations and surface plated on solid YPD medium for counting colonies. Three replicates were used. Additional replicates were used when irradiation resulted in low counts. Plates were incubated for 48 hours at 30°C. Survival curves were generated from experimental data by plotting doses of radiation versus Log N/N0 (where N is the number of colony forming units [CFUs] at a given doses and N0 is the negative control number of CFU).
Yeast Alkaline Comet Assay
To observe the microgels without overlapping comets, the OD was adjusted to an OD600 of approximately 0.4. Cells were harvested (1 mL) by centrifugation at 5000 rpm and 4°C for 5 minutes and washed twice with the same volume of S buffer (1 mol/L sorbitol, 25 mmol/L KH2PO4, pH 6.5). The cells were resuspended in 500 μL S buffer, and 1 μL β-mercaptoethanol and 50 μL lyticase (0.5 U/μL) were added at 37°C for 1 hour to obtain spheroplasts. Spheroplasts were collected by centrifugation at 5000 rpm at 4°C for 5 minutes, followed by washing with the same volume of ice-cold S buffer. The comet assay was performed according to the protocol adopted for yeast cells with optimization and modifications.21–23 The pellets were resuspended carefully in 500 μL low-melting agarose (1.5%; wt/vol in S buffer) at 35°C. Then, 40 μL of this mixture was spread over a slide coated with a water solution of 0.5% normal-melting agarose, covered with a cover slip, and placed on ice to solidify. After cover slips were removed, the slides were incubated in lysis buffer (30 mmol/L NaOH, 1 mol/L NaCl, 0.05% laurylsarcosine, 50 mmol/L EDTA, and 10 mmol/L Tris-HCl, pH 10) for 20 minutes at 4°C. Subsequently, the slides were submerged in electrophoresis buffer (30 mmol/L NaOH, 10 mmol/L EDTA, and 10 mmol/L Tris-HCl, pH 10) for 20 minutes at 4°C. The samples were electrophoresed in the same buffer for 8 minutes at 0.5 V/cm. After electrophoresis, the slides were incubated in a neutralization buffer (10 mmol/L Tris-HCl, pH 7.4) for 10 minutes, followed by consecutive incubations in 76% and 96% ethanol, both for 5 minutes at room temperature. The slides were air-dried and stained with 20 μL ethidium bromide (2 mg/mL), covered with cover slips, and analyzed by fluorescence microscopy (Olympus BX61, Tokyo, Japan) at 400× magnification. Fifty representative comets per slide and 6 slides per sample were analyzed.
In order to quantify DNA damage, the images were analyzed by 2 methods. In the first method, the comets were classified into 5 stages (1-5) according to the length and the fluorescence intensity of the comet tail. Stage 1 (no tail) and stage 2 (halo around the nucleus) corresponded to cells without significant DNA damage. Stages 3 to 5 corresponded to a gradual increase in DNA damage.24–26 Fifty comets per slide were randomly selected, and the percentage of comets in each stage was estimated for each slide.26 The comet score was calculated using the following formula:
| 1 |
Here, the arbitrary units (AUs) for stages 1 through 5 were defined as 0, 100, 200, 300, and 400, respectively. In the second method, the extent of DNA damage was quantified using the CometScore freeware, which considered the indices of tail length (TL) and olive tail moment (OTM).
Data Analysis
The measured data were expressed as the mean (standard deviation), and the fitting of equations was performed using OriginPro 8.0 software.
Results
Yeast Cell Inactivation Due to X-Rays or CI Exposure
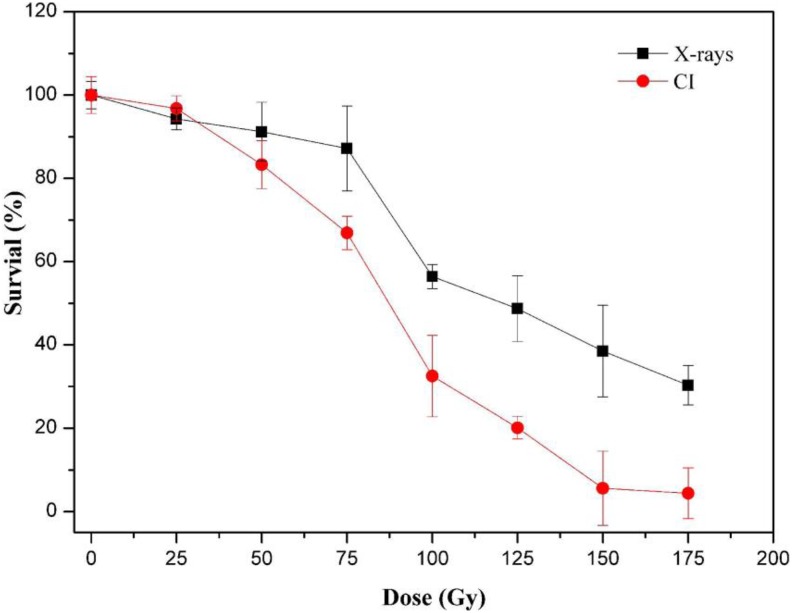
As presented in Figure 1, when exposed to CI, the cell survival rate exhibited an apparent negative relationship with dose, and the rate of decrease was accelerated for doses higher than 50 Gy. However, the survival of cells changed minimally from 150 Gy to 175 Gy. When cells were treated with X-rays, the trend in cell survival rate was similar to that observed for CI, but CI was more lethal than X-rays at identical doses.
Figure 1.
Survival curves of yeast cells with 0, 25, 50, 75, 100, 125, 150, and 175 Gy CI (diamonds) or X-rays (squares) radiation. Each point represents the mean (standard deviation) of 3 estimates. The nonirradiated sample (0 Gy) was used as the negative control. CI indicates carbon ion.
Relationship Between Radiation Dose and DNA Damage
Many studies have indicated that the conditions of the comet assay, such as the time of lysis, unwinding, electrophoresis, and voltage, influenced the sensitivity and estimation of the results. Furthermore, the various quantification indices also affected the sensitivity and detection limits of DNA damage.17 Hence, in this study, the experimental conditions were optimized to ensure sensitive detection of DNA damage and minimal DNA damage in the control group (0 Gy). The optimal parameters for lysis time, unwinding time, and electrophoresis time were 10, 20, and 8 minutes, respectively, and the appropriate voltage was 0.5 V/cm (data not shown).
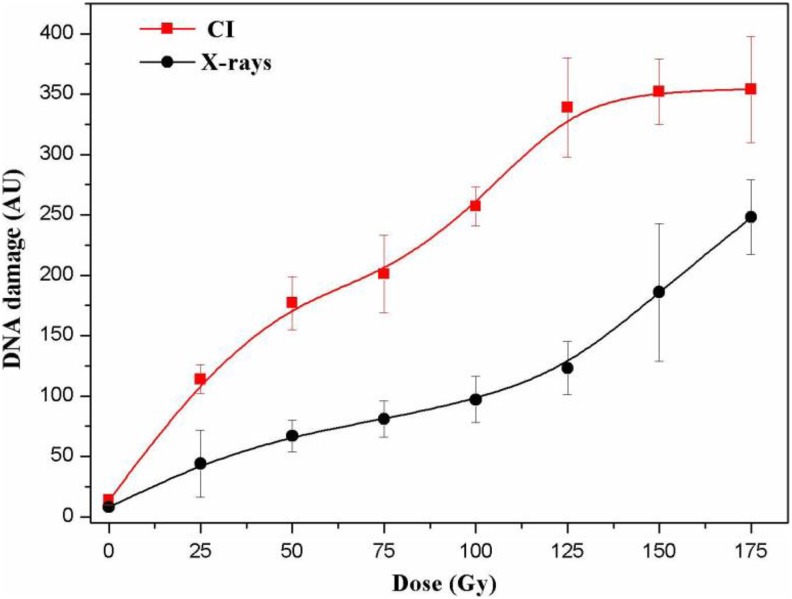
The comet score was used to quantify DNA damage in yeast cells. The stages were established according to the length and fluorescence intensity of the comet tails. The results are shown in Figure 2. The degree of DNA damage was measured by the comet score and expressed as AUs. We analyzed DNA damage by the comet assay immediately after CI or X-rays radiation. Yeast cells revealed a dose-dependent increase in the comet score after X-rays radiation from 25 to 175 Gy and with CI radiation from 25 to 125 Gy, and this damage was significant (Figure 3). However, DNA damage (based on the comet score) did not differ for CI radiation doses that exceeded 125 Gy. Therefore, the quantitative comet score cannot be used to evaluate extensive DNA damage in yeast cells.
Figure 2.
Classification of the comets. Comet categories are defined by the size and fluorescence intensity of the head and tail as well as tail length. The differences among stages reflect the extent of DNA damage. Stage 1, normal nucleus (very little DNA damage); stage 2, halo around the nucleus (slight DNA damage); stages 3 to 5, gradual increase in the length and intensity of the comet tail in parallel with a decrease in nuclear DNA content (varying degrees of DNA damage).
Figure 3.
Comet score (AU) indicating DNA damage in yeast cells after CI or X-rays irradiation. DNA damage was evaluated in an alkaline comet assay and quantified as described in the material and methods. Data are expressed as the mean AU of 3 independent experiments; bars indicate standard deviations. AU indicates arbitrary units; CI, Carbon ion.
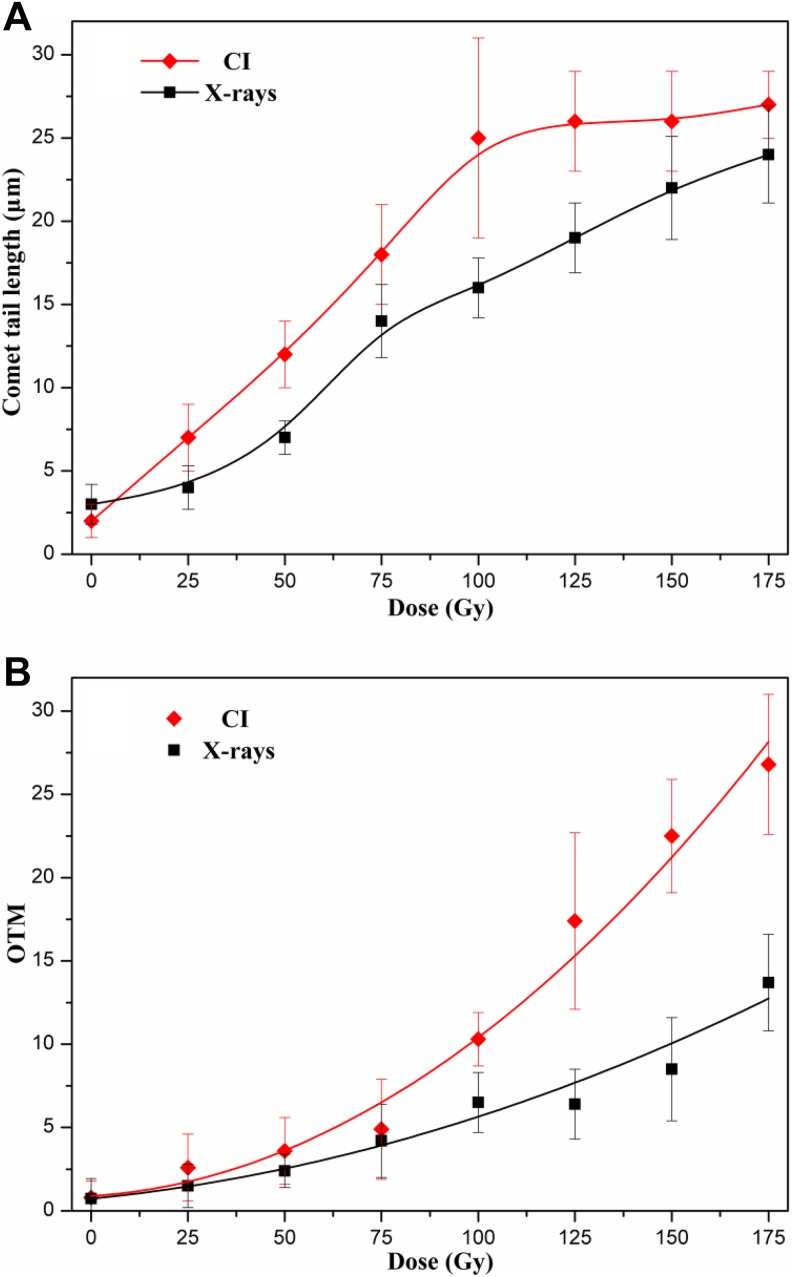
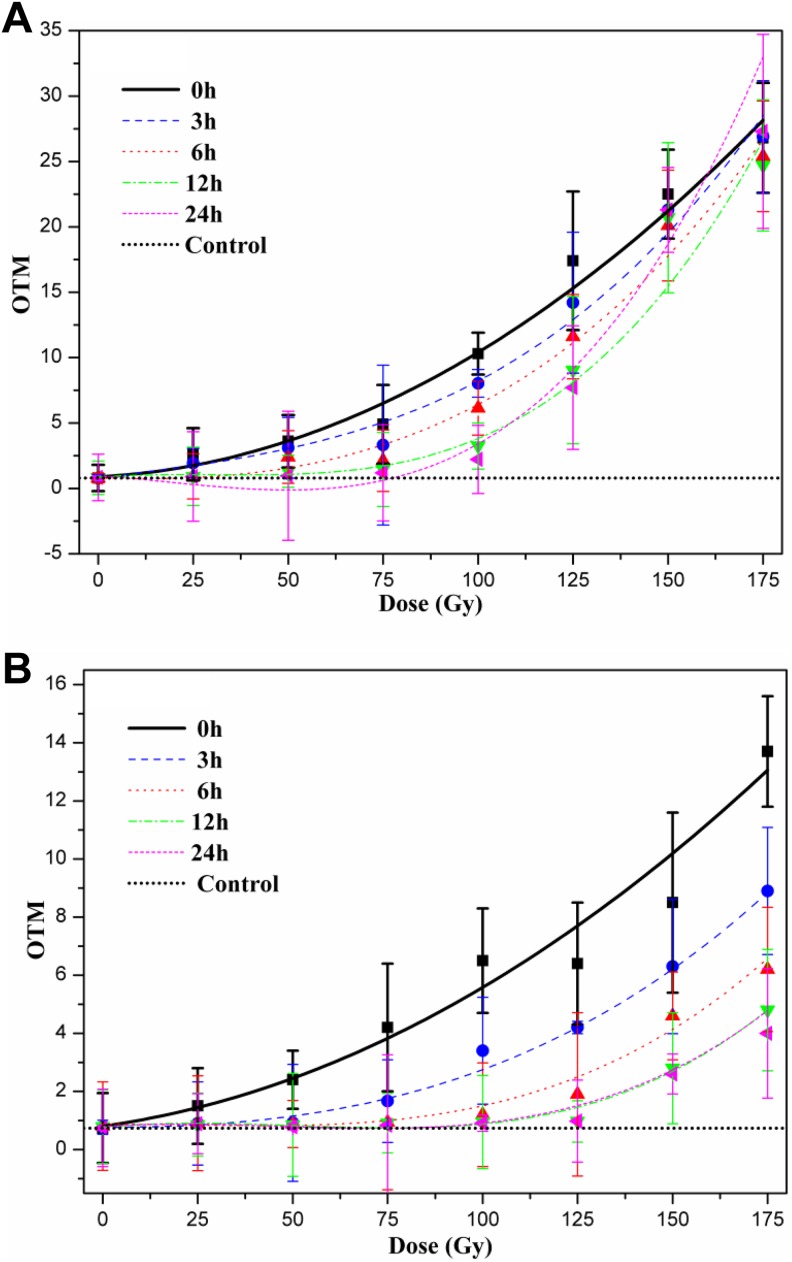
Subsequently, quantification using CometScore was performed and the estimated parameters were compared (Figure 4) to determine the optimal index. The results indicated that TL easily arrived at a plateau. The dose-dependent relationship was not apparent for CI radiation >125 Gy. The sensitivity of TL was higher than that of OTM. However, OTM could approximate the extent of DNA damage induced by CI or X-rays radiation in the range of 25 to 175 Gy. Therefore, OTM was the only indicator confirmed in the subsequent assays. Models of the relationship between CI or X-rays radiation dose and DNA damage (OTM) were fitted by various functions. As shown in Table 1, the quadratic function fitted the data best for both types of radiation and had the highest coefficient. The results suggested that the relationship between yeast DNA damage induced by CI or X-rays radiation and the dose was best described by the quadratic function. On the other hand, the cells with DNA damage gradually increased based on visual images of comets (Figure 5), and the extent of DNA damage was accelerated with an increase in dose (Figure 4B).
Figure 4.
The quantification of DNA damage in yeast cells irradiated with various doses CI or X-rays using the Freeware Cometscore. Mean (SD) for TL (A) and OTM (B) of 3 independent experiments, with at least 300 comets per experiment for each dose (50 comets per slide). CI, Carbon ion; OTM, olive tail moment; SD, standard deviation; TL, tail length.
Table 1.
The Fitted Equations of Different Function Models for OTM (Y) in the Comet Assay and Dose (X) of CI or X-Rays Radiation.a
| Models of Function | Fitted Equations | |
|---|---|---|
| CI | X-Rays | |
| Linear function | Y = 0.1566X − 2.5600; R 2 = 0.9243 | Y = 0.0672X − 0.2417; R 2 = 0.9068 |
| Exponential function | Y = 1.1966e 0.0195X; R 2 = 0.8435 | Y = 1.0319e 0.0155X; R 2 = 0.8971 |
| Quadratic function | Y = 0.0008X 2 + 0.0202X + 0.7583; R 2 = 0.9815 | Y = 0.0003X 2 + 0.0233X + 0.8575; R 2 = 0.9691 |
| Cubic function | Y = −4.9E−6X 3 + 0.0020X 2 − 0.0600X + 1.5409; R 2 = 0.8993 | Y = 3.4E − 6X 3 − 0.0006X 2 + 0.0725X + 0.4035; R 2 = 0.9179 |
Abbreviations: CI, Carbon ion; OTM, olive tail moment.
a The dose–effect relationships for DNA damage from CI or X-rays were fitted by a linear function, exponential function, quadratic function, and cubic function. R 2 indicates the fitting coefficient.
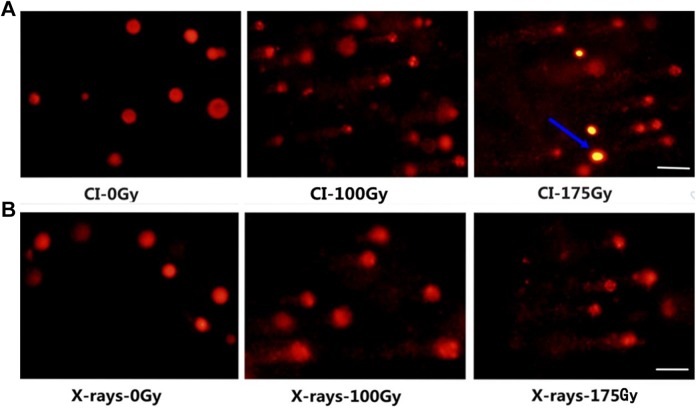
Figure 5.
Image samples obtained by the application of the yeast comet assay for untreated cells (0 Gy) and cells treated with CI (A) or X-rays (B) radiation of 100 and 175 Gy. The images were acquired with fluorescence microscopy at ×400 magnification and correspond to a representative assay from 3 independent experiments. Here, the blue arrow represents the cells that did not form comets (ie, did not form protoplasts); white bar = 20 μm. CI indicates carbon ion.
DNA Damage at Different Repair Times
To analyze the relationship between DNA damage induced by CI or X-rays radiation and dose for different repair times, the dose–effect functions were studied at 3, 6, 12, and 24 hours. The results demonstrated that the overall dose–effect curves for different repair times are fitted to the cubic function and could be summarized by the formula Y OTM = aX 3 + bX 2 + cX + d (Y represents the measured OTM values and X represents the doses of CI or X-rays radiation; data not shown). The coefficients of the cubic function (a, b, c, and d) are determined by the repair time. Based on Figure 6A, the extent of DNA damage was apparently diminished for longer repair times when cells were irradiated with CI in the range of 25 to 125 Gy, but the degree of decrease differed depending on the dose. In particular, the DNA repair ability was extremely weak for doses greater than 125 Gy. For exposure to X-rays, the trend varied considerably compared to CI exposure (Figure 6B). The extent of repair was greater in response to X-rays than to CI for the same repair time, suggesting that the DNA damage resulting from X-rays was easier to repair. Although the DNA damage decreased to varying extents before 12 hours, it barely changed from 12 to 24 hours, indicating that the repair of DNA damage was already nearly complete. Moreover, more repair of DNA damage was observed after treatment with X-rays compared to CI at identical doses.
Figure 6.
Relationship between radiation dose and DNA damage for various repair times. DNA damage of yeast cells treated by CI or X-rays radiation was repaired in normal growth conditions (30°C and 200 rpm/min) for 3, 6, 12, and 24 hours, respectively, and then the residual DNA damage was assessed by OTM. A, For CI radiation, the dose–effect curves for various repair times was fitted by the cubic function and the extent of repair of DNA damage decreased for increasing doses. B, For X-rays radiation, the dose–effect curves for various repair times were also fitted to a cubic function, but the DNA damage was almost completely repaired in the range of 25 to 175 Gy. CI indicates carbon ion; OTM, olive tail moment.
Repair of DNA Damage
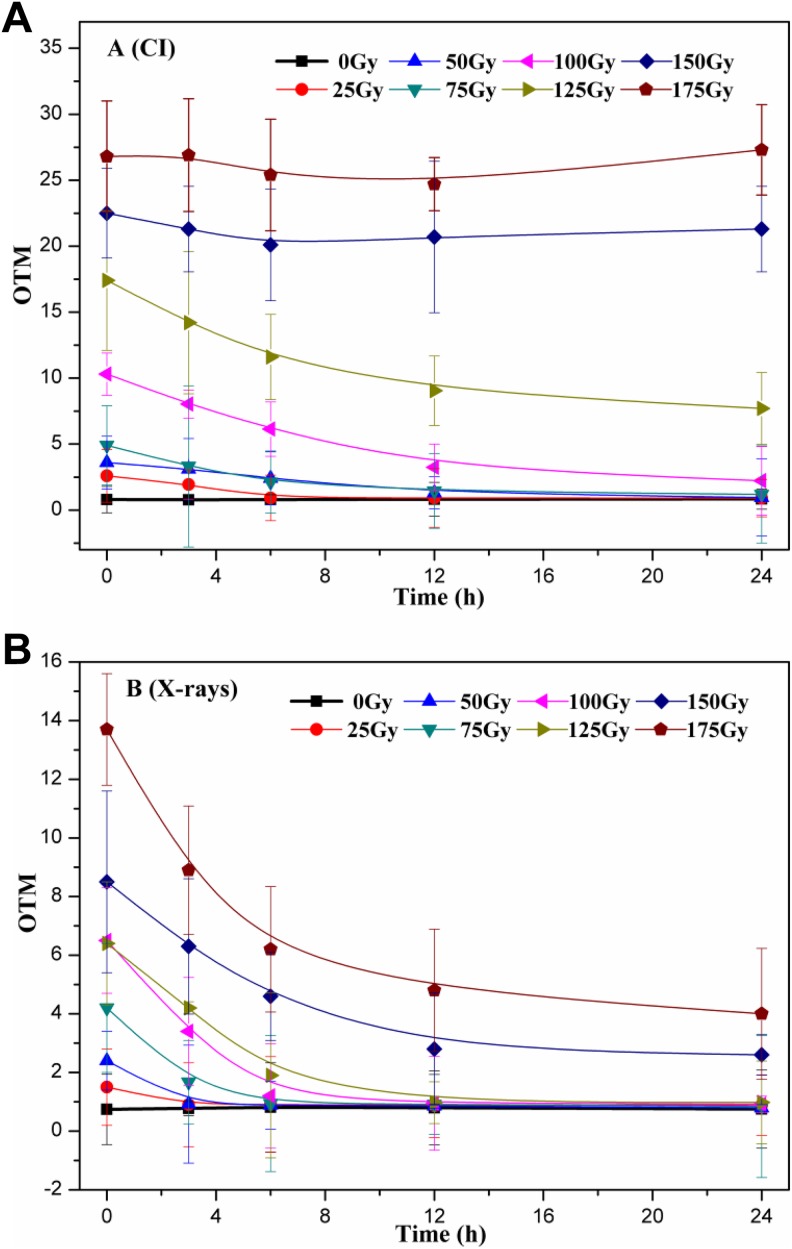
Cells were irradiated with various doses of CI or X-rays and incubated in normal growth conditions (30°C and 200 rpm) for 3, 6, 12, and 24 hours to allow damaged DNA to be repaired before performing the comet assay. As shown in Figure 7A, OTM decreased more gradually as repair time increased when the CI radiation doses were <125 Gy, compared to the corresponding control (0 hours). In particular, the DNA damage was completely repaired for small doses of radiation (<100 Gy) within 6 hours, and the repair time required differed. However, for doses >125 Gy, the extent of DNA damage did not change with increased repair time. The kinds of DNA damage have already exceeded the repair ability of yeast cells. Furthermore, after X-ray radiation, DNA damage was nearly repaired for doses ranging from 25 to 175 Gy, and only the time required and the extent of attained repair differed according to the dose (Figure 7B). The rate of DNA repair gradually diminished as the dose increased, and the decrease in OTM (DNA damage) was dependent on the initial OTM (results not shown). For example, although the DNA damage was fully repaired within 3 hours for the 25 Gy treatment, only approximately 70.8% of the DNA damage was repaired at 24 hours, and the repair limit was nearly reached for the 175 Gy treatment. Therefore, yeast cells were able to decrease the DNA damage induced by CI or X-rays radiation in a dose-dependent manner.
Figure 7.
Repair of DNA damage induced by CI or X-rays radiation in yeast cells. A, Cells were irradiated with various doses of CI before embedding in agarose and lysing, and then incubated in normal growth conditions (30°C, 200 rpm/min) for 3, 6, 12, and 24 hours to repair. B, Cells were irradiated with various doses of X-rays following the methods in (A). The data represented are the means of at least 3 independent experiments. CI indicates carbon ion.
Moreover, the models of DNA repair with irradiation for various CI or X-rays doses were fitted within the repairable range and are listed in Table 2. Other than the lack of repair observed for 150 and 175 Gy CI radiation within 24 hours and the finished repair for exposure to less than 75 Gy X-rays radiation within 3 hours, the kinetics process for DNA repair was fitted to a quadratic function, suggesting that the decline in DNA damage was not uniform (ie, nonlinear) with respect to repair time. However, overall, it appeared to be rapid at first and slowed over time.
Table 2.
Functional Models of DNA Repair With CI or X-Rays Radiation of Various Doses.
| Dose (Gy) | Functional Models | |
|---|---|---|
| CI | X-Rays | |
| 25 | Y = 0.0074X 2 − 0.2433X + 2.5109; R 2 = 0.8990 | <3 hours |
| 50 | Y = 0.0063X 2 − 0.2670X + 3.7112; R 2 = 0.9910 | <3 hours |
| 75 | Y = 0.0127X 2 − 0.4474X + 4.6812; R 2 = 0.9700 | <3 hours |
| 100 | Y = 0.0207X 2 − 0.8349X + 10.333; R 2 = 0.9998 | Y = 0.0236X 2 − 0.7677X + 5.8889; R 2 = 0.9064 |
| 125 | Y = 0.0262X 2 − 1.0205X + 17.165; R 2 = 0.9943 | Y = 0.0217X 2 − 0.7338X + 6.1752; R 2 = 0.9682 |
| 150 | Nonrepaira | Y = 0.0200X 2 − 0.7186X + 8.3708; R 2 = 0.9964 |
| 175 | Nonrepaira | Y = 0.0330X 2 − 1.1516X + 12.842; R 2 = 0.9466 |
Abbreviation: CI, carbon ion.
a Nonrepair: barely any repair for the corresponding doses; <3 hours: repair nearly complete within 3 hours for the corresponding doses.
Discussion
In this study, yeast cells were irradiated with X-rays or CI beams and the relationships between the doses and DNA damage and repair were analyzed. According to the results, CI exposure had a markedly stronger lethal effect than X-rays exposure (Figure 1). This may indicate that high-LET IR led to more serious DSBs, which were difficult to repair.27,28 An alkaline comet assay was performed to evaluate the dose–effect relationship for the DNA damage and repair processes. Although yeast cells as a model organism are suitable for this method, the studies are limited.18,20–23,29–36 A possible reason is that the amount of DNA in yeast cells is considerably lower than in higher eukaryotes. The haploid yeast cell nucleus contains only approximately 13 Mb DNA, whereas human cell nuclei contain approximately 3300 Mb DNA. Furthermore, the cell wall adds complexity to the comet assay because it must be broken. In fact, the cell wall is thick and strong, and the preparation of protoplasm causes exterior DNA damage more easily. Nevertheless, in this assay, lyticase was used to degrade yeast cell walls surrounding the protoplasm,36,37 and this allowed the comet assay to be performed on yeast21–23 and optimized. Our results suggest that 10-minute alkali lysis and 20 minutes unwinding followed by 8 minutes of electrophoresis (0.5 V/cm) represent the optimal conditions for the detection of DNA damage in yeast cells exposed to CI or X-ray radiation. Quantitative results were obtained (Figure 5), and the images of comets were clearer than those obtained in the previous studies.29–31 Therefore, the difficulties associated with the use of yeast haploid cells for comet assays were overcome. Control cells (0 Gy) prepared for the comet assay displayed comet-like features (not data shown), consistent with previous assays. Probably, the DNA was damaged by handling the cells during the experimental conditions, or/and there was initial DNA damage present or replication forks. To enhance the comparison among radiation groups, the experimental conditions were controlled to the greatest extent possible so that the DNA damage in the control group (0 Gy) was minimal. In order to quantify the DNA damage using the comet assay, the images were analyzed by 2 methods. Comets were scored using standard stages and using the CometScore, which considered TL and OTM. Although both methods are frequently used for the quantification of comet assays,24–26 the distinction between them and their applicability is not clear. A comparative study of the method was carried out in these experiments. For the former method, standard comet images of varying grades were obtained (Figure 2), and the dose–effect relationship for DNA damage quantified by the comet score was estimated (Figure 3). No differences in sensitivity to CI radiation were observed when the dose of CI radiation was >125 Gy (the lethality rate was greater than 80%), suggesting that this quantification method was not acceptable for assessing DNA damage. Thus, comet scores are only suitable when DNA damage is mild. Using CometScore to assess DNA damage, adaptive parameters were selected for TL and OTM. The results indicated that TL arrived at a plateau more easily. The dose-dependent relationship was not observed when the dose of CI radiation exceeds 125 Gy. The sensitivity of TL was higher than that of OTM (Figure 4A). Tail length is appropriate at relatively low damage levels, and the tail increases in intensity but not in length for higher degrees of damage.24,38 However, OTM, a sensitive parameter for the comet assay using a single-celled species with a uniform DNA damage pattern, could be used to evaluate a wider array of DNA damage. Thus, these results suggest that OTM could be considered when selecting appropriate parameters for the exact assessment of DNA damage induced by IR in the yeast comet assay.
The relationship between irradiation dose and DNA damage has been investigated since the comet assay was established, and obvious dose–effect relationships have been found in tumor cells and germ cells.39–41 The great majority of dose–effect relationships are approximated by a linear function.17,42,43 However, in the present experiments, the quadratic function best fitted the dose–effect after CI or X-rays radiation in yeasts, based on the form and the fitting coefficient (Figure 4B; Table 1). These results demonstrate that the dose–effect relationship depends on cell type.
There are few reports examining the models that fit dose–effect curves for different repair times.44 The relationships observed in our experiments are displayed in Figure 6 and precisely fit a cubic function. Differences between the radiation types and the levels of DNA repair were observed in the present study. In the case of X-ray radiation with a low LET, DNA damage mainly occurred in SSBs which is easy to repair. On the contrary, CI exposure leads to irreparable DSBs. Hence, the decline in DNA damage induced by X-rays is markedly faster than in damage induced by CI, along with the repair times, and the dose–effect curves followed a cubic function for each repair time.
In addition, studies on the repair of DNA damage induced by IR have focused on the patterns of repair and key proteins. The comet assay which can measure residual micro-DNA damage is appropriate for the study of DNA repair.45 In fact, the mechanisms of DNA damage and repair are simultaneously activated once live cells are subjected to IR, and the rate and mechanisms of speedy repair are unlike those of slow repair. In the radiation assay, it is extremely difficult to obtain samples immediately (0 hours), and the yeast cells that are detected have accomplished rapid DNA repair. Therefore, the DNA repair investigated is mainly a slow DNA repair. A kinetic model was creatively used to examine the process of DNA repair in this study. The results indicate that quadratic functions accurately describe the process within the repairable range (Figure 7), and corresponding equations can be used to roughly evaluate the process and time necessary for DNA repair (Table 2). However, if DNA damages are minimal, the repairs are accomplished rapidly (X-rays <100 Gy). In contrast, DNA damages are too serious for repair when the CI doses are >125 Gy. Based on the curves, the estimate of DNA damage at each time point can be calibrated, and the DNA damage values after radiation can be inferred (0 hours). Therefore, these results help clarify the process of DNA repair and facilitate accurate calibration of DNA damage estimates.
In conclusion, the present study demonstrates the utility of the comet assay optimized for detecting DNA damage caused by CI or X-rays in yeast cells. Regarding the 2 quantification methods studied, neither TL nor the comet score were suitable for assessing DNA damage induced by high doses of CI. In contrast, OTM, as a universal parameter that could measure extensive DNA damage, was suited to this experiment. The dose–effect relationships for DNA damage after CI or X-rays radiation were fitted to quadratic functions. For various repair times, cubic functions were equally appropriate. Furthermore, quadratic functions were applied to the DNA repair process. To our knowledge, this is the first time that the comet assay has been applied to assess the yeast DNA damage and repair processes using kinetic functions. As yeast is a model organism, these method and concept may be extensively applied to studies on DNA integrity.
Acknowledgments
The authors would like to thank the colleagues at HIRFL for providing the high-quality carbon ion beams.
Authors’ Note: Miaomiao Zhang and Guozhen Cao contributed equally to this work and should be considered cofirst authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Chinese Academy of Sciences Key Deployment Project (No. KFZD-SW-109), Joint project of Chinese Academy of Sciences and Industrial Technology Research Institute (CAS-ITRI 201801), and the National Natural Science Fund of China (No. 11575259).
References
- 1. Pouget JP, Douki T, Richard MJ, Cadet J. DNA damage induced in cells by γ and UVA radiation as measured by HPLC/GC-MS and HPLC-EC and comet assay. Chem Res Toxicol. 2000;13(7):541–549. [DOI] [PubMed] [Google Scholar]
- 2. Ohnishi T, Mori E, Takahashi A. DNA double-strand breaks: their production, recognition, and repair in eukaryotes. Mutat Res. 2009;669(1-2):8–12. [DOI] [PubMed] [Google Scholar]
- 3. Hirano T, Kazama Y, Ohbu S, et al. Molecular nature of mutations induced by high-LET irradiation with argon and carbon ions in Arabidopsis thaliana. Mutat Res Fundam Mol Mech Mutagen. 2012;735(1-2):19–31. [DOI] [PubMed] [Google Scholar]
- 4. Mcmahon SJ, Currell FJ. A robust curve-fitting procedure for the analysis of plasmid DNA strand break data from gel electrophoresis. Radiat Res. 2011;175(6):797–805. [DOI] [PubMed] [Google Scholar]
- 5. Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):79–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–374. [DOI] [PubMed] [Google Scholar]
- 7. Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417(3):639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lieber MR. The mechanism of double-strand DNA break repair by the non-homologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore S, Stanley FK, Goodarzi AA. The repair of environmentally relevant DNA double strand breaks caused by high linear energy transfer irradiation-no simple task. DNA Repair. 2014;17(5):64–73. [DOI] [PubMed] [Google Scholar]
- 10. Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2005;1(1):23–29. [DOI] [PubMed] [Google Scholar]
- 11. Olive PL. Impact of the comet assay in radiobiology. Mutat Res. 2009;681(1):13–23. [DOI] [PubMed] [Google Scholar]
- 12. Speit G, Hartmann A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage. Methods Mol Biol. 2006;314:275–286. [DOI] [PubMed] [Google Scholar]
- 13. Gradzka W, Iwanenko T. A non-radioactive, PFGE-based assay for low levels of DNA double-strand breaks in mammalian cells. DNA Repair. 2005;4(10):1129–1139. [DOI] [PubMed] [Google Scholar]
- 14. Olive PL, Banath JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res. 1990;122(1):86–94. [PubMed] [Google Scholar]
- 15. Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123(1):291–298. [DOI] [PubMed] [Google Scholar]
- 16. Collins AR, Dusinska M, Gedik CM, Stĕtina R. Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect. 1996;104(suppl 3):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ueno S, Kashimoto T, Susa N, et al. Assessment of DNA damage in multiple organs of mice after whole body X-irradiation using the comet assay. Mutat Res. 2007;634(1-2):135–145. [DOI] [PubMed] [Google Scholar]
- 18. Reliene R, Pollard JM, Sobol Z, Trouiller B, Gatti RA, Schiestl RH. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat Res. 2009;665(1-2):37–43. [DOI] [PubMed] [Google Scholar]
- 19. Wirzinger G, Weltje L, Gercken J, Sordyl H. Genotoxic damage in field collected three-spined sticklebacks (Gasterosteus aculeatus L.): a suitable biomonitoring tool? Mutat Res. 2007;628(1):19–30. [DOI] [PubMed] [Google Scholar]
- 20. Rank J, Syberg K, Jensen K. Comet assay on tetraploid yeast cell. Mutat Res. 2009;673(1):53–58. [DOI] [PubMed] [Google Scholar]
- 21. Azevedo F, Marques F, Fokt H, Oliveira R, Johansson B. Measuring oxidative DNA damage and DNA repair using the yeast comet assay. Yeast. 2011;28(1):55–61. [DOI] [PubMed] [Google Scholar]
- 22. Litwin I, Bocer T, Dziadkowiec D, Wysocki R. Oxidative stress and replication-independent DNA breakage induced by arsenic in Saccharomyces cerevisiae . Plos Genet. 2013;9(7):e1003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oliveira R, Johansson B. Quantitative DNA damage and repair measurement with the yeast comet assay. Methods Mol Biol. 2012;920:101–109. [DOI] [PubMed] [Google Scholar]
- 24. Collins AR. The comet assay for DNA damage and repair. Mol Biotechnol. 2004;26(3):249–261. [DOI] [PubMed] [Google Scholar]
- 25. DobrzyåSka MM. DNA damage in organs of female and male mice exposed to nonylphenol, as a single agent or in combination with ionizing irradiation: a comet assay study. Mutat Res. 2014;772(18):14–19. [DOI] [PubMed] [Google Scholar]
- 26. Driessens N, Versteyhe S, Ghaddhab C, et al. Hydrogen peroxide induces DNA single-and double-strand breaks in thyroid cells and is therefore a potential mutagen for this organ. Endocr Relat Cancer. 2009;16(3):845–856. [DOI] [PubMed] [Google Scholar]
- 27. Magnander K, Elmroth K. Biological consequences of formation and repair of complex DNA damage. Cancer Lett. 2012;327(1-2):90–96. [DOI] [PubMed] [Google Scholar]
- 28. Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res. 2008;49(3):203–210. [DOI] [PubMed] [Google Scholar]
- 29. Miloshev G, Mihaylov I, Anachkova B. Application of the single cell gel electrophoresis on yeast cells. Mutat Res. 2002;513(1):69–74. [DOI] [PubMed] [Google Scholar]
- 30. Lah B, Gorjanc G, Nekrep FV, Marinsek-Logar R. Comet assay assessment of wastewater genotoxicity using yeast cells. Bull Environ Contam Toxicol. 2004;72(3):607–616. [DOI] [PubMed] [Google Scholar]
- 31. Nemavarkar PS, Chourasia BK, Pasupathy K. Detection of γ-irradiation induced DNA damage and radioprotection of compounds in yeast using comet assay. J Radiat Res. 2004;45(2):169–174. [DOI] [PubMed] [Google Scholar]
- 32. Raspor P, Plesnicar S, Gazdag Z, et al. Prevention of intracellular oxidation in yeast: the role of vitamin E analogue, Trolox (6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxyl acid). Cell Biol Int. 2005;29(1):57–63. [DOI] [PubMed] [Google Scholar]
- 33. Staneva D, Peycheva E, Georgieva M, Efremov T, Miloshev G. Application of comet assay for the assessment of DNA damage caused by chemical genotoxins in the dairy yeast Kluyveromyces lactis. Antonie Van Leeuwenhoek. 2013;103(1):143–152. [DOI] [PubMed] [Google Scholar]
- 34. Banerjee P, Talapatra SN, Mandal N, et al. Genotoxicity study with special reference to DNA damage by comet assay in fission yeast, Schizosaccharomyces pombe exposed to drinking water. Food Chem Toxicol. 2008;46(1):402–407. [DOI] [PubMed] [Google Scholar]
- 35. Marques F, Azevedo F, Johansson B, Rui O. Stimulation of DNA repair in Saccharomyces cerevisiae by Ginkgo biloba leaf extract. Food Chem Toxicol. 2011;49(6):1361–1366. [DOI] [PubMed] [Google Scholar]
- 36. Grzelak A, Macierzynska E, Bartosz G. Accumulation of oxidative damage during replicative aging of the yeast Saccharomyces cerevisiae . Exp Gerontol. 2006;41(9):813–818. [DOI] [PubMed] [Google Scholar]
- 37. Catalfo A, Calandra ML, Renis M, Serrentino ME, De Guidi G. Rufloxacin induced photosentization in yeast. Photochem Photobiol Sci. 2007;6(2):181–189. [DOI] [PubMed] [Google Scholar]
- 38. Kent CRH, Eady JJ, Ross GM, et al. The comet moment as a measure of DNA damage in the comet assay. Int J Radiat Biol. 1995;67(6):655–660. [DOI] [PubMed] [Google Scholar]
- 39. Wada S, Karahayashi H, Kobayashi Y, et al. The relationship between cellular radiosensitivity and radiation-induced DNA damage measured by the comet assay. J Vet Med Sci. 2003;65(4):471–477. [DOI] [PubMed] [Google Scholar]
- 40. Qiu LM, Li WJ, Pang XY, et al. Observation of DNA damage of human hepatoma cells irradiated by heavy ions using comet assay. World J Gastroenterol. 2003;9(7):1450–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hail GA, Hendry JH, Daniel CP, Morris ID. Germ cell and dose-dependent DNA damage measured by the comet assay in murine spermatozoaa after testicular X-irradiation. Biol Reprod. 2002;67(3):854–860. [DOI] [PubMed] [Google Scholar]
- 42. Singh NP.Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res. 2000;455(1):111–127. [DOI] [PubMed] [Google Scholar]
- 43. Risom L, Møller P, Vogel U, Kristjansen PE, Loft S., Loft S.X-ray-induced oxidative stress: DNA damage and gene expression of HO-1, ERCC1 and OGG1 in mouse lung. Free Radic Res. 2003;37(9):957–966. [DOI] [PubMed] [Google Scholar]
- 44. Neumaier T, Swenson J, Pham C, et al. Evidence for formation of DNA repair centers and dose-response nonlinearity in human cells. Proc Natl Acad Sci U S A. 2012;109(2):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garaj-Vrhovac V, Kopjar N, Raem D, Vekić B, Miljanić S, Ranogajec-Komor M. Application of the alkaline comet assay in biodosimetry: assessment of in vivo DNA damage in human peripheral leukocytes after a gamma radiation incident. Radiat Prot Dosim. 2002;98(4):407–416. [DOI] [PubMed] [Google Scholar]