Abstract
Purpose:
The purpose of this study was to evaluate pupillary light reflexes (PLRs) mediated by rod, cone, and intrinsically photosensitive retinal ganglion cell pathways as indices of outer- and inner-retinal function in patients who have enhanced S-cone syndrome (ESCS) due to NR2E3 mutations.
Methods:
Four patients with ESCS (ages 16-23 years) participated in the study. Subjects were tested with long- and short-wavelength single-flash full-field ERG stimuli under light adapted conditions. They were also tested with an established pupillometry protocol involving 1-second duration, long- and short-wavelength stimuli under dark- and light-adapted conditions. The PLR was measured as a function of stimulus luminance. Transient PLRs were measured under all conditions, and sustained PLRs were measured under the highest luminance dark-adapted condition.
Results:
Two-color light-adapted full-field ERGs demonstrated larger amplitude responses for short-wavelength stimuli relative to long-wavelength stimuli of the same photopic luminance, with 3 of 4 ESCS patients having super-normal a-wave amplitudes to the short-wavelength stimulus. B/A wave ratios were reduced in all four cases. Transient PLRs elicited by low luminance stimuli under dark-adapted conditions (rod-mediated) were unrecordable, whereas the sustained PLRs elicited by high luminance stimuli (melanopsin-mediated) were normal. Cone-mediated PLRs were recordable for all four patients, but generally lower than normal in amplitude. However, the cone-mediated PLR was larger for the short-wavelength stimulus compared to the photopically matched long-wavelength stimulus at high luminances, a pattern that was not observed for control subjects. None of the PLR conditions demonstrated “super-normal” responses.
Conclusions:
ESCS patients appear to have generally well-preserved cone- and melanopsin-mediated PLRs, indicating intact inner-retinal function. Two-color pupillometry demonstrates greater sensitivity to short-wavelength light under higher-luminance conditions and could complement the ERG as a tool for evaluating retinal function in ESCS.
Keywords: Enhanced S-cone syndrome, Pupillary light reflex, Pupillometry, Full-field electroretinogram, NR2E3
Introduction
Enhanced S-cone syndrome (ESCS) is an autosomal recessive retinal dystrophy caused by mutations in the NR2E3 gene (OMIM 604485).1 NR2E3 codes for a retina-specific nuclear transcription factor involved in development and maintenance of photoreceptors.2,3 Loss of the regulatory function of the NR2E3 protein leads to an overabundance of short-wavelength cones (S-cones).1,4,5,6 In humans with ESCS, the excess S-cones are thought to replace photoreceptors that otherwise would have developed into rods in the presence of NR2E3.4,7
ESCS patients exhibit congenital night blindness, which is attributable to a lack of (or minimal) functional rods.8,9,10 They also demonstrate progressive visual loss11,12 due to both outer retinal (photoreceptor) degeneration, which occurs primarily along the arcades, and cystoid maculopathy.1 When ESCS is associated with more advanced vitreo-retinal degeneration it may also be referred to by an older eponym, Goldmann-Favre syndrome (GFS).13
The full-field electroretinogram (ERG), using clinical protocols such as the International Society for Clinical Electrophysiology of Vision (ISCEV) standard protocol,14 shows specific abnormalities in ESCS, including a lack of rod-mediated responses, similar waveforms elicited by high-luminance, single-flash stimuli under dark- and light-adapted conditions that have delayed implicit times, and variably reduced 30-Hz flicker amplitudes.11,15,16 Non-standard experimental ERGs using short-wavelength stimuli elicit responses of much larger amplitude compared to photopically matched long-wavelength stimuli,5,8,16 as well as super-normal amplitudes to short-wavelength stimuli under photopic conditions.
In addition to the increased number of S-cones, ESCS patients are also thought to have an increased number of S-cone retinal ganglion cells (i.e. S − [L + M] retinal ganglion cells; RGCs). Furthermore, the L and M-cone inputs into the S-cone RGCs appear to be attenuated. These alterations in post-receptor mechanisms likely play a role in the psychophysically-measured enhanced sensitivity to short-wavelength light and increased S-cone visual acuity.6
Pupillometry provides an alternative approach to study receptoral and post-receptoral function in patients with ESCS. The pupil response is complex in that it can be rod-, cone-, or melanopsin-mediated. That is, the rod and cone pathways provide input into the intrinsically photosensitive RGCs (ipRGCs) that contain the photopigment melanopsin and control the pupil response. Adaptation conditions and stimuli can be varied to selectively target these pathways,17 permitting assessment of both inner- and outer-retinal function. The extent to which the response of the pupil is affected by the increase in S-cones is unknown, but it is possible that ipRGCs have altered synaptic input, like the S-cone RGCs discussed above. Thus, the primary purpose of this study was to evaluate pupillary light reflexes (PLRs) mediated by the rod, cone, and melanopsin pathways as indices of outer- and inner-retinal function in patients with ESCS. We were particularly interested in determining the extent to which short- and long-wavelength stimuli elicit pupil constrictions, given the large disparity between short-and long-wavelength sensitivity assessed psychophysically and the amplitude differences assessed by ERG.
Methods
Four unrelated patients with ESCS participated in the study (ESCS1-ESCS4; ages 16, 18, 20 and 23 years). All four were diagnosed by a specialist in inherited retinal diseases (GAF) based on history, clinical examination and clinical ERG findings. Goldmann visual fields had been performed in all four subjects at clinical visits within the past 3 years, and all four previously had clinical full-field ERGs performed on various instruments. All four patients were either homozygous or compound heterozygous for disease-associated variants of the NR2E3 gene (Table 1).
Table 1.
Enhanced S-cone syndrome patient demographics, genotypes, and visual acuities
| Patient # | Age (years) | Gender | NR2E3 mutations | Snellen visual acuity |
|---|---|---|---|---|
| ESCS1 | 16 | M | IVS1-2 A>C and Ala102Asp GCC>GAC | OD 20/20-1; OS 20/15-3 |
| ESCS2 | 18 | F | IVS1-2 A>C and Ser269del2tggTC | OD 20/200-1; OS 20/60-2 |
| ESCS3 | 20 | M | IVS1-2 A>C homozygous | OD 20/25-1; OS 20/70-1 |
| ESCS4 | 23 | F | IVS1-2 A>C and Arg311Gln CGG>CAG | OD 20/40; OS 20/25 |
| ERG controls; n = 10 | 24 - 38 (range) | 6 F / 4 M | ||
| Pupillometry controls; n = 6 | 19 - 34 (range) | 3 F / 3 M |
The four ESCS patients complained of longstanding, stable nyctalopia. Central vision complaints were varied, and best-corrected visual acuities ranged from 20/15 to 20/200. ESCS1, ESCS2 and ESCS3 had substantially normal Goldmann visual fields to either the V4e or III4e target. ESCS4 demonstrated constriction of the Goldmann visual field to the V4e target. All four had fundus changes consistent with ESCS and/or GFS,13,16 including hypopigmentation along the arcades in all cases. ESCS1 had subtle whitish spots along the arcades, while ESCS2 and ESCS3 had a few small round pigment clumps along the arcades. ESCS4 could be classified as having Goldmann-Favre syndrome based on posterior subcapsular cataracts, prominent round pigment clumps along the arcades, and dendritic whitish lesions in the periphery. At the time of the study, ESCS1-ESCS3 were all being treated with carbonic anhydrase inhibitors for cystoid macular edema; both ESCS1 and ESCS3 were under treatment with topical dorzolamide 2% three times per day in each eye, and ESCS2 was under treatment with oral extended release acetazolamide 500 mg per day.18,19,20 ESCS4 had minimal microcystic changes in the macula and was not under treatment for macular edema.
Electroretinography
All four ESCS patients and ten visually-normal subjects (age range 24-38) underwent a short full-field ERG protocol involving two stimuli under light-adapted conditions with a Burian-Allen electrode lens in one eye. All subjects were dilated with phenylephrine 2.5% and tropicamide 1% prior to ERG testing. The peak ±half-bandwidth at half-height of the short-wavelength (blue) stimulus was 444 ± 8 nm, and the long-wavelength (red) stimulus was 632 ± 10 nm. The single flash stimulus luminance was 4.5 photopic cd s m−2 and the stimulus was presented on a 30 cd m−2 6500 K white background generated by a Diagnosys ColorDome Ganzfeld (Diagnosys, LLC, Lowell, MA, USA). Signal frequencies below 0.312 and above 300 Hz were filtered out by the instrument. At least three separate responses to each flash were obtained and averaged for both conditions.
Pupillometry
The four ESCS patients and six visually-normal subjects (mean age 28 years, range 19-34) underwent full-field pupillometry. Full-field pupillometry stimuli of 1-second long luminance pulses were generated by a Diagnosys ColorDome Ganzfeld, with narrow-band LEDs of 465 ± 13 nm (short-wavelength, blue), and 642 ± 11 nm (long wavelength, red). The peak wavelengths are different than those shown above because different ColorDome units were used for the pupillometry and ERG measurements. The PLR was measured with a plastic frame-mounted infrared camera system (Arrington Research, Scottsdale, AZ), at a sampling rate of 60 Hz.21 The fellow eye was patched. An intensity series of dark adapted responses were elicited by long- and short-wavelength stimuli ranging from −4 log cd m−2 (0.0001 cd m−) to 2.65 log cd m−2 (450 cd m−2), in 1 log steps for all but the highest luminance stimulus, after 10 minutes of dark adaptation. The transient PLRs were defined as the maximum constriction relative to baseline pupil diameter (prior to light onset). A melanopsin response was measured with the 2.65 log cd m−2 short-wavelength stimulus as the median pupil diameter between 5 and 7 seconds after the stimulus offset, relative to baseline.22 The direct measurement approach (stimulation and recording of the same eye) has been shown to provide reliable, repeatable responses.23 An intensity series of light-adapted responses were elicited by stimuli ranging from −1 log cd m−2 to 2.65 log cd m−2, using both long- and short-wavelength stimuli on a rod-suppressing short-wavelength background of 0.78 log cd m−2 (6 cd m−2), with transient PLRs measured as described above.
Results
Electroretinography
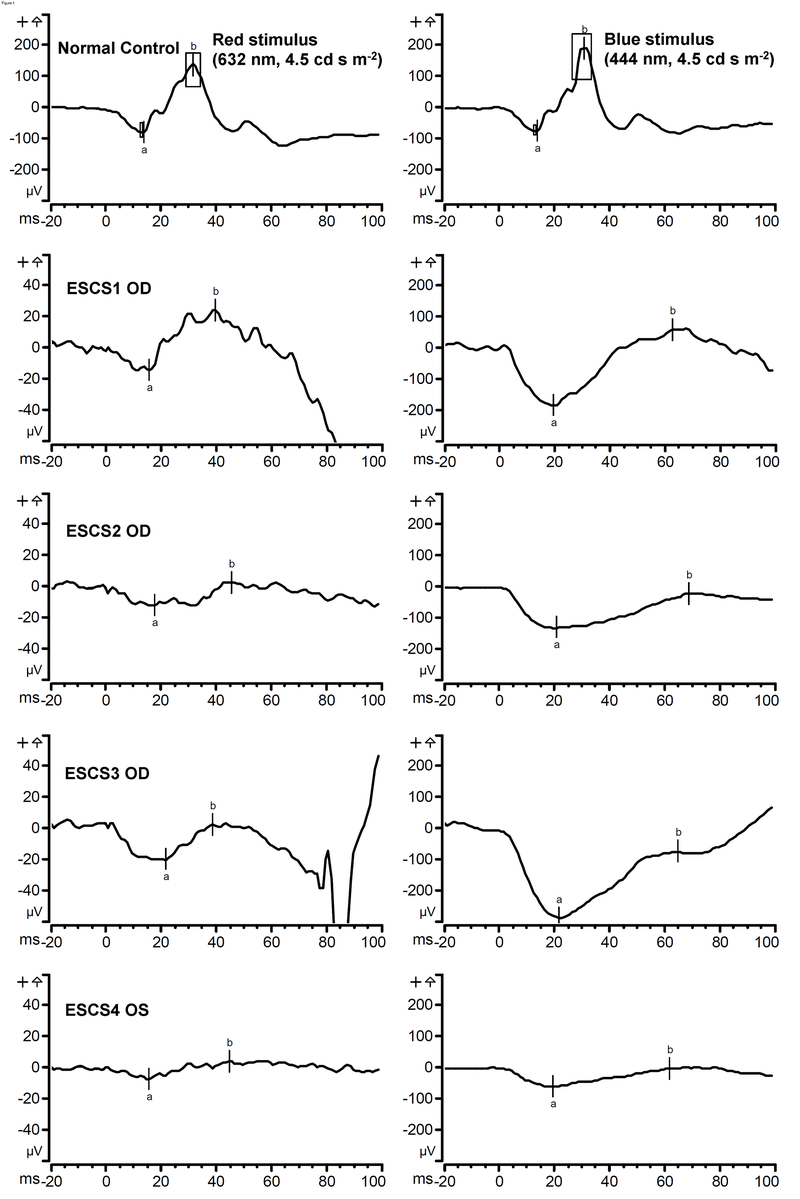
In all four ESCS patients, clinical full-field ERGs had been performed at previous visits on various instruments. Rod-isolated responses had been found to be non-detectable. The high-luminance flash presented under scotopic and/or photopic conditions had prolonged b-wave implicit times in all cases. With the narrow-band photopic stimuli used in this study, the responses of our ESCS patients were pathognomonic for this condition, as well as consistent with previous studies that used similar two-color stimuli.1,16 For example, all four ESCS patients demonstrated much larger amplitudes to the short-wavelength stimulus than to the long-wavelength stimulus (Table 2 and Figure 1). To the long-wavelength stimulus, their b-waves were considerably smaller in amplitude compared to normal, with implicit times that appeared to be delayed, although the implicit times could not be measured with certainty due to the small amplitudes of the responses. ESCS2’s and ESCS4’s responses to the long-wavelength stimulus were nearly non-detectable. To the short-wavelength stimulus, ESCS1-ESCS3 had a-waves that were of larger amplitude than the normal range, whereas ESCS4’s a-wave amplitude was near the lower range of normal. Short-wavelength stimulus b-wave amplitudes were normal in ESCS1 and ESCS3, but sub-normal in ESCS2 and ESCS4. In all cases the a- and b-waves were delayed relative to the normal range, with the b-wave implicit times approximately twice as long as normal (27–34 ms for normal controls and 62–69 ms for ESCS patients). Such delayed implicit times under photopic conditions are consistent with S-cone mediation of the ERG in the ESCS patients.16 Also, in all ESCS cases with the short-wavelength stimulus, the b/a wave ratio was considerably reduced, most prominently in the case of ESCS3.
Table 2.
Enhanced S-cone syndrome full-field ERG and pupillometry amplitudes
| Light adapted ffERG a-wave/b-wave (μV) |
Pupillometry transient constriction |
Pupillometry sustained |
|||||
|---|---|---|---|---|---|---|---|
| Patient # | Eye tested | Red 4.5 cd s m−2 stimulus | Blue 4.5 cd s m−2 stimulus | −3 log cd m−2 blue dark adapted (Rod) | 2.6 log cd m−2 red light adapted (Cone) | 2.6 log cd m−2 blue light adapted (Cone) | 2.6 log cd m−2 blue dark adapted (Melanopsin) |
|
|
|
|
|
||||
| ESCS1 | OD | 14 / 39 | 183 / 247 | Non-detectable | 46% | 49% | 37% |
| ESCS2 | OD | 12 / 15 | 130 / 110 | Non-detectable | 26% | 36% | 47% |
| ESCS3 | OD | 20 / 23 | 287 / 213 | Non-detectable | 26% | 34% | 44% |
| ESCS4 | OS | 7 / 12 | 59 / 60 | Non-detectable | 29% | 41% | 41% |
| ERG controls | 46 - 94 / 146 - 261 | 55 - 92 / 168 - 316 | |||||
| Pupillometry controls | 22% - 40% | 37% - 55% | 38% - 55% | 32% - 56% | |||
Abbreviations: ffERG = full-field electroretinogram; see Methods for stimulus and background wavelengths Pupillometry stimuli are all 1 second duration
The pupillary response is defined as percent relative constriction: [1 − (minimum pupil size) / (baseline pupil size)]×100%
Control data listed are ranges; see Methods for stimulus and background wavelengths
Figure 1.
Two-color light-adapted single-flash 4.5 photopic cd s m−2 electroretinogram with a 30 cd m−2 white (6500K ) background. Panels on the left show responses to the long-wavelength (red) stimulus, and panels on the right show responses to the short-wavelength (blue) stimulus. The top two panels show example responses from a normal subject, with boxes indicating the range of a- and b-wave amplitudes and implicit times obtained from normal subjects. Note the similarity between the long- and short-wavelength normal responses, due to primarily (L/M) cone mediation. The bottom eight panels show responses from the four ESCS subjects (ESCS1-ESCS4); the vertical scale for the four ESCS patients has been adjusted relative to that of the normal subject in order to make the small amplitude responses more visible. Long-wavelength ESCS a- and b-wave amplitudes were sub-normal. ESCS amplitudes are considerably larger for the short-wavelength stimulus. Short-wavelength ESCS a-wave amplitudes were larger than normal in all but ESCS4. B-wave amplitudes were variable, but all ESCS b-wave implicit times for the short-wavelength stimulus were consistently prolonged in all four cases.
Pupillometry
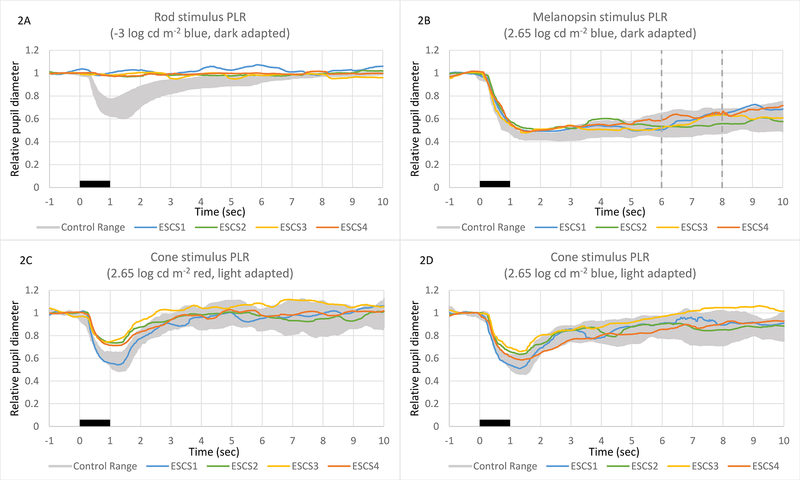
Figure 2 shows the ESCS patients’ PLRs obtained under conditions previously reported to elicit rod- (A), melanopsin- (B), and cone-mediated (C,D) responses.17,22 In each panel, the normal control range is represented by the gray region. For the rod and cone conditions, the normal PLR is characterized by a transient constriction (peak latency < 2 sec) followed by a relatively rapid return to the baseline. The PLRs of the ESCS patients were non-detectable under the rod-mediated condition. The melanopsin-mediated response of the normal subjects is characterized by a prolonged constriction following the offset of the stimulus that lasts for several seconds (Fig. 2B). The two vertical dashed lines in this panel indicate the time range over which the sustained response was measured. The ESCS patients’ melanopsin-mediated PLRs were all within the normal range. The ESCS patients’ light-adapted long-wavelength stimulus cone condition (Fig. 2C) responses were sub-normal, and their light-adapted short-wavelength stimulus cone condition (Fig. 2D) responses were around the lower range of normal. ESCS1 was an exception, in that his light-adapted PLRs were more normal (higher amplitude) than the rest of the ESCS patients for both wavelengths. The ESCS light adapted PLR amplitudes were larger for the short-wavelength stimulus than for the long-wavelength stimulus (paired t-test P = 0.023 for ESCS vs. P = 0.23 for controls). The mean dark-adapted baseline pupil size for the controls (6.41 mm) did not differ significantly from that of the patients (6.58 mm; t = 0.28, p = 0.78). Similarly, the mean light-adapted baseline pupil size for the controls 3.78 mm) did not differ significantly from that of the patients (3.83 mm; t = 0.17, p = 0.87).
Figure 2.
Pupillometry traces showing relative PLR constriction to three stimuli proposed to have clinical utility. Stimuli under all conditions were 1-second in duration, starting at time zero (indicated by a black bar). In all three panels, the range of normal responses is represented by a gray zone, and the responses of the four ESCS patients are each shown separately. Transient PLR amplitudes (2A and 2B) were measured at the point of maximal constriction. ESCS transient PLRs were non-detectable under the dark-adapted −3 log cd mx2212;2 blue stimulus “rod condition” (2A). ESCS transient PLRs were around the lower range of normal to mildly sub-normal under the light-adapted 1 log cd m−2 red stimulus “cone condition” (2B). Sustained PLRs were measured at 5 to 7 seconds after the stimulus offset (demarcated by vertical lines in 2C). ESCS sustained PLRs were well within the normal range under the dark-adapted 2.65 log cd m−2 blue stimulus “melanopsin condition” (2C).
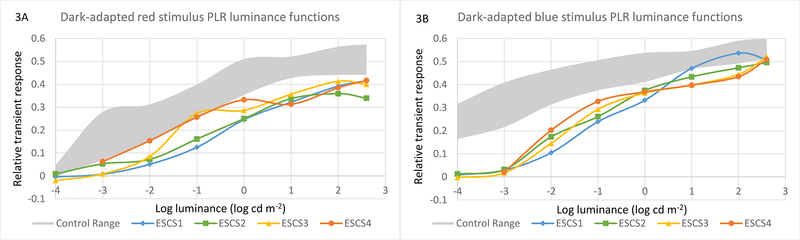
Figure 3 shows the dark-adapted transient PLR amplitudes for long- (3A) and short- (3B) wavelength stimuli, obtained for a series of stimulus luminance levels, with data shown for each ESCS patient, along with the normal control range (gray region). Note that the “rod condition” shown in Fig. 2A (−3 log cd m−2) corresponds to the second lowest luminance in Fig. 3B. The patients’ PLRs tended to be moderately reduced, compared to the controls, for all luminance levels when measured with the long-wavelength stimulus (Fig. 3A). In contrast, the patients’ PLRs were non-detectable for low-luminance short-wavelength stimuli (c.f. Fig. 2B), but only slightly reduced compared to controls at high luminance levels. In summary, the patients had marked abnormalities for low-luminance, short-wavelength stimuli (presumably rod-mediated in visually-normal subjects), with generally mild deficits under all other transient PLR conditions.
Figure 3.
Pupillary light reflex luminance-response functions. In both panels, the range of normal responses is represented by a gray zone. The two panels show dark-adapted PLRs for long-wavelength (red, 3A) and short-wavelength (blue, 3B) stimuli, tested at −4, −3, −2, −1, 0, 1, 2, and 2.65 log cd m−2. In 3A, the ESCS PLRs are moderately sub-normal for the long-wavelength stimulus across the luminance range. In 3B, the ESCS PLRs are non-detectable at the lowest ranges (attributable to a lack of rod function), and approach the lower limit of the normal range as the luminance increases.
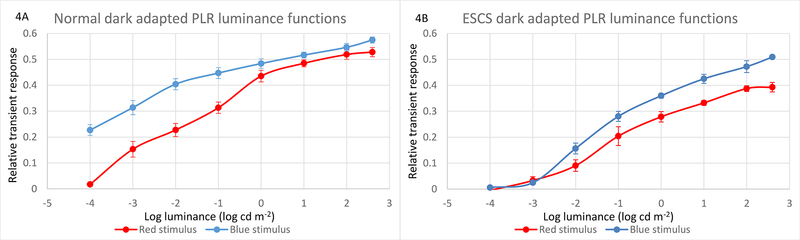
To provide additional insight into possible explanations for the pattern of data shown in Fig. 3, the mean PLRs for the controls and ESCS patients were computed and are plotted in Figure 4 as a function of log stimulus luminance. This figure allows direct comparisons of the dark-adapted PLRs measured with long- and short-wavelength stimuli for the controls (4A) and patients (4B). Under dark-adapted conditions in control subjects, the PLR elicited by low luminance short-wavelength stimuli is larger than that elicited by long-wavelength stimuli of the same photopic luminance. For example, a short-wavelength −4.0 log cd m−2 flash elicited a pupil constriction of 23%; the luminance of a long-wavelength stimulus had to be approximately 2 log units higher (−2 log cd m−2) in order to achieve a similarly sized PLR, indicating primarily rod-pathway mediation for the low luminance levels. At high luminances, the control PLRs were nearly equivalent for the long- and short-wavelength stimuli, indicating primarily L and M cone mediation of the PLRs. These results are consistent with previous publications.17,22 A “maximum constriction” driven by the most intense blue flash in the dark (2.72 mm; normal average) did not approach the mechanical limitation of the pupil, as it was still a larger diameter than the constriction driven by the high luminance red flash against the rod suppressing background (2. 16 mm).
Figure 4.
Pupillary light reflex luminance-response functions, replotted with averaged responses, allowing direct comparison of long- to short-wavelength stimuli. The dark-adapted luminance-response function in normal controls (4A, top left) shows a 2 log unit greater sensitivity to short- versus long-wavelength stimuli at the lower luminances, consistent with rod mediation of those low luminance responses. At higher luminances, the dark-adapted normal responses to long- and short-wavelength stimuli nearly overlap, consistent with saturation of the rod component and the increasing influence of cones on the transient responses. The dark-adapted luminance-response function in ESCS patients (4B, top right) show a lack of responses at the lowest luminances, and consistently greater relative sensitivity to the short-wavelength stimuli at all luminances above threshold.
The PLR results obtained from the ESCS patients were substantially different. That is, there was no measurable pupil response for either wavelength at low luminances (−4.0 and −3.0 log cd m−2) and there was an essentially constant vertical shift in the luminance functions for higher stimulus luminances. These results suggest that there is no (or negligible) rod-mediated PLR and that the high-luminance (presumably cone-mediated) short- and long-wavelength stimuli are not photopically equivalent for the ESCS patients.
As mentioned above, both dark-adapted rod-isolating ERGs and dark-adapted rod-mediated condition PLRs (e.g. at −3 log cd m−2) were non-detectable in all four ESCS subjects. Although photopic ERGs (Fig. 1) and light-adapted PLRs (Fig. 2C and 2D) were detectable in all four cases, clear correlations between the two tests were generally not found. One possible correlation was seen where the patient with the largest amplitude ERG b-wave amplitudes (ESCS1, Fig. 1) also had the largest light-adapted PLR amplitudes (blue traces in Fig. 2C and 2D), but the other three patients had PLR amplitudes that were closely clustered together.
Discussion
The primary purpose of this study was to evaluate PLRs mediated by rod, cone, and intrinsically photosensitive retinal ganglion cell pathways as indices of outer- and inner-retinal function in ESCS patients. Two-color ERGs were also obtained from the patients for comparison to the pupillometry data.
The protocol of short-wavelength versus long-wavelength photopic ERG used in this study has been well established in ESCS.16 Our use of these stimuli was meant to demonstrate a known effect in ESCS (confirming the “enhanced S-cone” phenotype) for comparison to the relevant PLRs. As expected, the ESCS ERG a- and b-wave amplitudes were much larger to the short-wavelength stimulus than to the long-wavelength stimulus, and implicit times were very prolonged compared to normal with the short-wavelength stimulus. Both of these effects can be attributed to the large S-cone contribution to the signal. B/A wave ratios were reduced in all four ESCS subjects, possibly related to the inner retinal changes that are seen in ESCS.24
The pattern of PLR abnormality was consistent among the four patients. Namely, the rod-mediated PLR was absent and the cone-mediated PLR showed substantial differences for photopically matched long-versus short-wavelength stimuli. The sustained PLRs under dark-adapted conditions (“melanopsin condition”) were normal for the ESCS patients, suggesting that ipRGC and optic nerve function is intact in these patients, as expected. This pattern of PLR abnormality is generally not found in other inherited retinal degenerative diseases, but it is not necessarily “pathognomonic” for ESCS. For example, patients with retinitis pigmentosa (RP) often lack a rod-mediated PLR and their cone-mediated PLR can vary, with some patients showing differences for photopically matched short- and long-wavelength stimuli.17,25 A non-recordable rod-mediated PLR has also been shown in some patients with Leber congenital amaurosis, but these patients typically have substantially reduced cone-mediated PLRs as well.17,26 Thus, while two-color pupillometry could have clinical value in ESCS (e.g. in a child, who may be uncooperative for ERG, with atypical ESCS fundus findings11,27,28,29), distinguishing among patients with RP and ESCS may be difficult on the basis of the PLR alone. Future modifications of the pupillometry backgrounds and stimuli, focusing on isolation of S-cones,30,31,32,33 could elicit more specific or perhaps “super-normal” PLRs from ESCS patients.
An interesting difference was observed between the long-wavelength light-adapted ERG and the PLR elicited by long-wavelength stimuli. Namely, the ERG amplitude was small (essentially non-detectable for two cases) (Fig. 1, left column), whereas the PLR was normal or mildly reduced (e.g. Fig. 2C). Recent work with various size pupillometry stimuli centered on the macula suggests that the cone-mediated aspect of the PLR is driven primarily by the central 16 degrees (diameter) or less.22 Also, studies using multifocal ERG in ESCS have found relative preservation of cone-mediated responses within the central 15 degrees of radius from fixation and reduction or absence of signal outside that radius.24 If the central macular cone function is relatively preserved in our subjects, then this would be sufficient to drive a near-normal L/M-cone mediated PLR in spite of a much reduced L/M-cone mediated full-field ERG.
Conclusion
To our knowledge, this study represents the first report of pupillometry findings in ESCS patients. We have demonstrated consistent differences between visually normal subjects and ESCS patients with a two-color full-field pupillometry protocol. Rod function was non-detectable with low-luminance stimuli under dark-adapted conditions, and the PLRs elicited by photopically matched short- and long-wavelength light were not equal. In comparison, ipRGC function appeared to be nearly normal under the PLR testing conditions used in this study.
Acknowledgments
Supported by: The Pangere Family Foundation, Gary, Indiana (GAF); National Institutes of Health Research grants EY019510 (JM) and EY001792 (UIC core grant) and an unrestricted departmental grant from Research to Prevent Blindness.
Funding:
The Pangere Family Foundation, Gary, Indiana (GAF), National Institutes of Health Research grants EY019510 (JM) and EY001792 (UIC core grant) and an unrestricted departmental grant from Research to Prevent Blindness provided financial support in the form of monetary funding.
The sponsors had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC (2000) Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 24(2):127–31. [DOI] [PubMed] [Google Scholar]
- 2.Webber AL, Hodor P, Thut CJ, Vogt TF, Zhang T, Holder DJ, Petrukhin K (2008) Dual role of Nr2e3 in photoreceptor development and maintenance. Exp Eye Res 87(1):35–48. [DOI] [PubMed] [Google Scholar]
- 3.Haider NB, Mollema N, Gaule M, Yuan Y, Sachs AJ, Nystuen AM, Naggert JK, Nishina PM (2009) Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res 89(3):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanda A, Swaroop A (2009) A comprehensive analysis of sequence variants and putative disease-causing mutations in photoreceptor-specific nuclear receptor NR2E3. Mol Vis 15:2174–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Hood DC, Cideciyan AV, Roman AJ, Jacobson SG (1995) Enhanced S cone syndrome: evidence for an abnormally large number of S cones. Vision Res 35(10):1473–81. [DOI] [PubMed] [Google Scholar]
- 6.Greenstein VC, Zaidi Q, Hood DC, Spehar B, Cideciyan AV, Jacobson SG (1996) The enhanced Scone syndrome: an analysis of receptoral and post-receptoral changes. Vision Res 36(22):3711–22. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Khan NW, Roger JE, Swaroop A (2011) Excess cones in the retinal degeneration rd7 mouse, caused by the loss of function of orphan nuclear receptor Nr2e3, originate from early-born photoreceptor precursors. Hum Mol Genet 20(21):4102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson SG, Marmor MF, Kemp CM, Knighton RW (1990) SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci 31(5):827–38. [PubMed] [Google Scholar]
- 9.Bonilha VL, Fishman GA, Rayborn ME, Hollyfield JG (2009) Retinal pathology of a patient with Goldmann-Favre syndrome. Ophthalmic Genet 30(4):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milam AH, Rose L, Cideciyan AV, Barakat MR, Tang WX, Gupta N, Aleman TS, Wright AF, Stone EM, Sheffield VC, Jacobson SG (2002) The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad Sci U S A 99(1):473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, Neveu MM, Hogg CR, Hunt DM, Moore AT, Bird AC, Webster AR, Holder GE (2008) Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci 49(5):2082–93. [DOI] [PubMed] [Google Scholar]
- 12.Pachydaki SI, Bhatnagar PA, Barbazetto IA, Klaver CC, Freund BK, Yannuzzi LA (2009) Long-term follow-up in enhanced s-cone syndrome. Retin Cases Brief Rep 3(2):118–20. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson SG, Román AJ, Román MI, Gass JD, Parker JA (1991) Relatively enhanced S cone function in the Goldmann-Favre syndrome. Am J Ophthalmol 111(4):446–53. [DOI] [PubMed] [Google Scholar]
- 14.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15.Vincent A, Robson AG, Holder GE (2013) Pathognomonic (diagnostic) ERGs. A review and update. Retina 33(1):5–12. [DOI] [PubMed] [Google Scholar]
- 16.Marmor MF, Jacobson SG, Foerster MH, Kellner U, Weleber RG (1990) Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol 110(2):124–34. [DOI] [PubMed] [Google Scholar]
- 17.Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC (2011) Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci 52(9):6624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genead MA, Fishman GA, McAnany JJ (2010) Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol 121(3):231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiszkielis M, Lubiński W, Penkala K (2013) Topical dorzolamide treatment of macular cysts in the enhanced S-cone syndrome patient. Doc Ophthalmol 126(3):241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iannaccone A, Fung KH, Eyestone ME, Stone EM (2009) Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol 147(2):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki A, Kardon RH (2007) Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol 27(3):195–204. [DOI] [PubMed] [Google Scholar]
- 22.Park JC, McAnany JJ (2015) Effect of stimulus size and luminance on the rod-, cone-, and melanopsin-mediated pupillary light reflex. J Vis 15(3):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei S, Goltz HC, Chandrakumar M, Wong AM (2015) Test-retest reliability of hemifield, central-field, and full-field chromatic pupillometry for assessing the function of melanopsin-containing retinal ganglion cells. Invest Ophthalmol Vis Sci 56(2):1267–73. [DOI] [PubMed] [Google Scholar]
- 24.Sustar M, Perovšek D, Cima I, Stirn-Kranjc B, Hawlina M, Brecelj J (2015) Electroretinography and optical coherence tomography reveal abnormal post-photoreceptoral activity and altered retinal lamination in patients with enhanced S-cone syndrome. Doc Ophthalmol 130(3):165–77. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki A, Crippa SV, Kardon R, Leon L, Hamel C (2012) Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Invest Ophthalmol Vis Sci 53(9):5562–9. [DOI] [PubMed] [Google Scholar]
- 26.Collison FT, Park JC, Fishman GA, McAnany JJ, Stone EM (2015) Full-Field Pupillary Light Responses, Luminance Thresholds, and Light Discomfort Thresholds in CEP290 Leber Congenital Amaurosis Patients. Invest Ophthalmol Vis Sci 56(12):7130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hull S, Arno G, Sergouniotis PI, Tiffin P, Borman AD, Chandra A, Robson AG, Holder GE, Webster AR, Moore AT (2014) Clinical and molecular characterization of enhanced S-cone syndrome in children. JAMA Ophthalmol 132(11):1341–9. [DOI] [PubMed] [Google Scholar]
- 28.Khan AO, Aldahmesh M, Meyer B (2007) The enhanced S-cone syndrome in children. Br J Ophthalmol 91(3):394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassiman C, Spileers W, De Baere E, de Ravel T, Casteels I (2013) Peculiar fundus abnormalities and pathognomonic electrophysiological findings in a 14-month-old boy with NR2E3 mutations. Ophthalmic Genet 34(1–2):105–8. [DOI] [PubMed] [Google Scholar]
- 30.Kimura E, Young RS (1999) S-cone contribution to pupillary responses evoked by chromatic flash offset. Vision Res 39(6):1189–97. [DOI] [PubMed] [Google Scholar]
- 31.Verdon W, Howarth PA (1988) The pupil’s response to short wavelength cone stimulation. Vision Res 28(10):1119–28. [DOI] [PubMed] [Google Scholar]
- 32.Cao D, Nicandro N, Barrionuevo PA (2015) A five-primary photostimulator suitable for studying intrinsically photosensitive retinal ganglion cell functions in humans. J Vis 15(1):15.1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitschan M, Jain S, Brainard DH, Aguirre GK (2014) Opponent melanopsin and S-cone signals in the human pupillary light response. Proc Natl Acad Sci U S A 111(43):15568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]