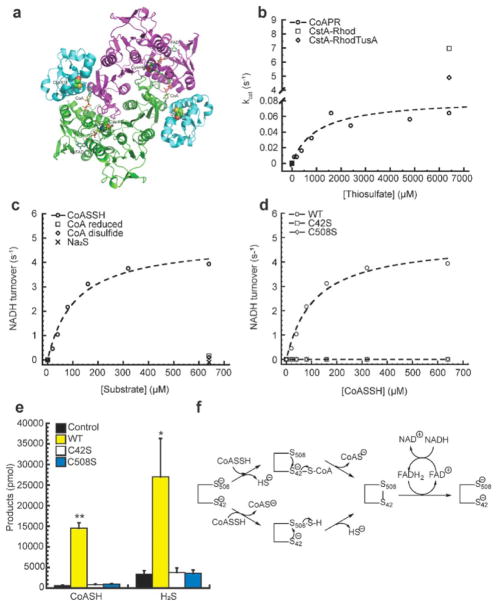
Figure 4.
Structural model and function of Ef CDR-RHD. (a) B. anthracis CDR-RHD adopts a homodimeric assembly state, shown in ribbon representation (PDB: 3ICT).36 The N-terminal CDR domains (residues 1–450) from two protomers are shaded in purple and green, respectively, with the RHD (residues 451–554) colored in cyan. Each CDR-RHD protomer binds to one CoA and one FAD molecule. Active site cysteine residues from CDR (C44; C42 in Ef CDR-RHD) and RHD (C514; C508 in Ef CDR-RHD) are highlighted as spheres. (b) The initial thiosulfate turnover rate as a function of thiosulfate concentration catalyzed by Ef CDR-RHD, S. aureus CstARhod, and CstARhodTusA, with the continuous fit to the Michaelis–Menten equation shows Ef CDR-RHD has minimal TST activity. Ef CDR-RHD Km determined as 1.0 ± 0.6 mM and Vmax determined as 0.08 ± 0.1 μmol·min−1·mg−1, and kcat is some 90-fold lower than CstARhod and CstARhodTusA.42 (c) The initial NADH turnover rate plotted as a function of the concentration of CoASSH, CoA, CoA disulfide, and Na2S, with the continuous line a fit to the Michaelis–Menten eq (Table 2) and demonstrating Ef CDR-RHD has authentic NADH and FAD-dependent persulfide reductase activity only toward CoASSH. (d) The initial NADH turnover rate plotted as a function of the concentration of wild-type (WT), C42S, and C508S CDR-RHDs, with the continuous lines fit to the Michaelis–Menten eq (Table 2) shows no detectable persulfide reductase activity for either cysteine mutant compared to wild-type. (e) Product analysis for persulfide activity of WT, C42S, and C508S CDR-RHDs where only WT shows an increase in both CoA and H2S products compared to C42S and C508S CDR-RHDs and control experiments without the addition of enzyme. Values represent mean ± SD derived from replicate experiments with statistical significance established using a paired t-test relative to control under the same conditions (**p ≤ 0.01, *p ≤ 0.05). (f) Possible models for Ef CDR-RHD as a coenzyme A persulfide reductase, CoAPR acting upon a single CoASSH molecule. Top route: C42 attacks the CoASSH to form a C42–S–S–CoA mixed disulfide with the release of HS−, and C508 then reduces the mixed disulfide with the release of CoA and formation of C42–S–S–C508 disulfide. Bottom route: C508 attacks the CoASSH to form an enzyme-bound persulfide with CoA, and then C42 attacks the C508 persulfide with the release of HS−, forming C42–S–S–C508 disulfide. In both cases, FADH2 eventually reduces the disulfide bond between C42 and C508, which is rereduced by NADH, and the enzyme turns over. An alternative model that incorporates features of the bottom route that invokes a CoA thiol as a cofactor has also been proposed.47