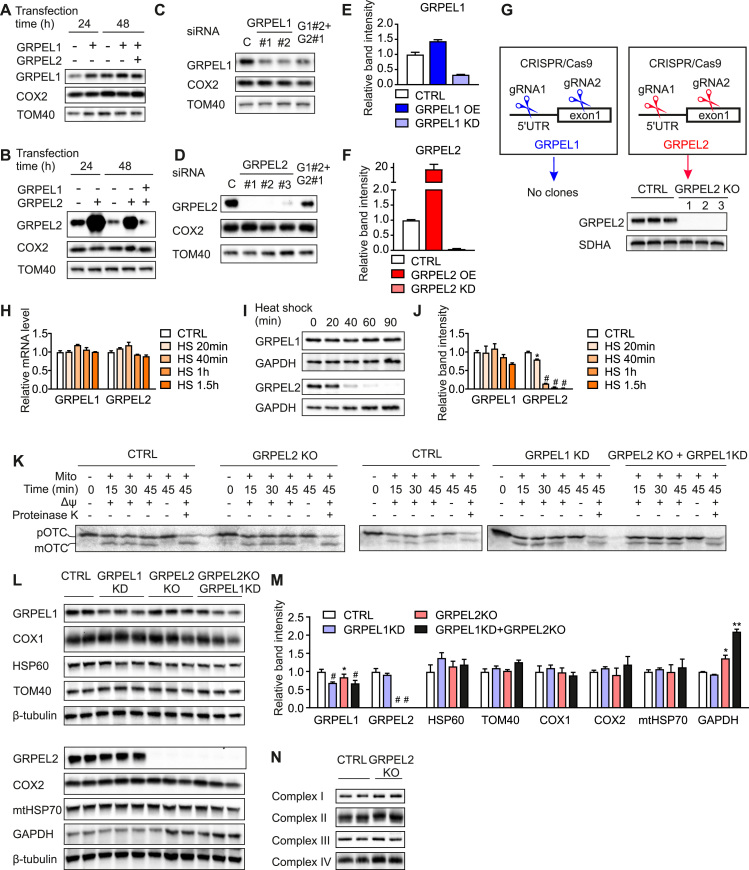
Fig. 2.
GRPEL2 is not essential for mitochondrial protein import in cultured cells A, B. Western blot analysis of 143B cells overexpressing GRPEL1, GRPEL2 or both. C, D. Western blot analysis of 143B cells depleted by siRNA for GRPEL1, GRPEL2 or both. G1, GRPEL1, G2, GRPEL2. E, F. Quantification of western blots showing the efficiency of GRPEL1 or GRPEL2 protein overexpression (OE) or knock down (KD) in 143B cells. The knock down or overexpression effect was analyzed after 48 h of transfection in comparison to TOM 40. G. CRISPR/Cas9 approach with guideRNAs (gRNA) was successful in generating several GRPEL2 knockout clones in HEK293 cells, but no GRPEL1 knockout clones were obtained. Western blot analysis shows full knockout of GRPEL2. SDHA was used as a loading control. H. mRNA levels of GRPELs in HEK293 cells exposed to heat shock as determined by qPCR assay (n = 4). I. Western blot analysis of HEK293 cells exposed to heat shock for indicated time. GAPDH was used as a loading control. J. Quantification of protein levels of GRPELs in HEK293 cells exposed to heat shock (n = 3). + 45 °C heat shock was used in all experiments. K. Import of [35S] preornithine transcarbamylase (pOTC) into isolated mitochondria of HEK293 cells. [35S] pOTC was incubated with mitochondria in the absence or presence of membrane potential (ΔΨ) for indicated time. As a control, after incubation with [35S] pOTC one sample was treated with proteinase K. Radiolabeled proteins were detected by phosphorimage analysis. Precursor (p) and mature matrix-processed (m) forms of OTC are indicated. L. Western blot analysis of HEK293 cells depleted for GRPEL1, GRPEL2 or for both. M. Quantification of protein levels determined by Western blots. β-tubulin was used as a loading control (n = 3). N. Blue native analysis of OXPHOS complexes in GRPEL2 KO cells. In all graphs data are presented as mean ± SD. CTRL, non-treated cells, *P < 0.05, #P < 0.0005 as compared to untreated cells (unpaired t-tests).