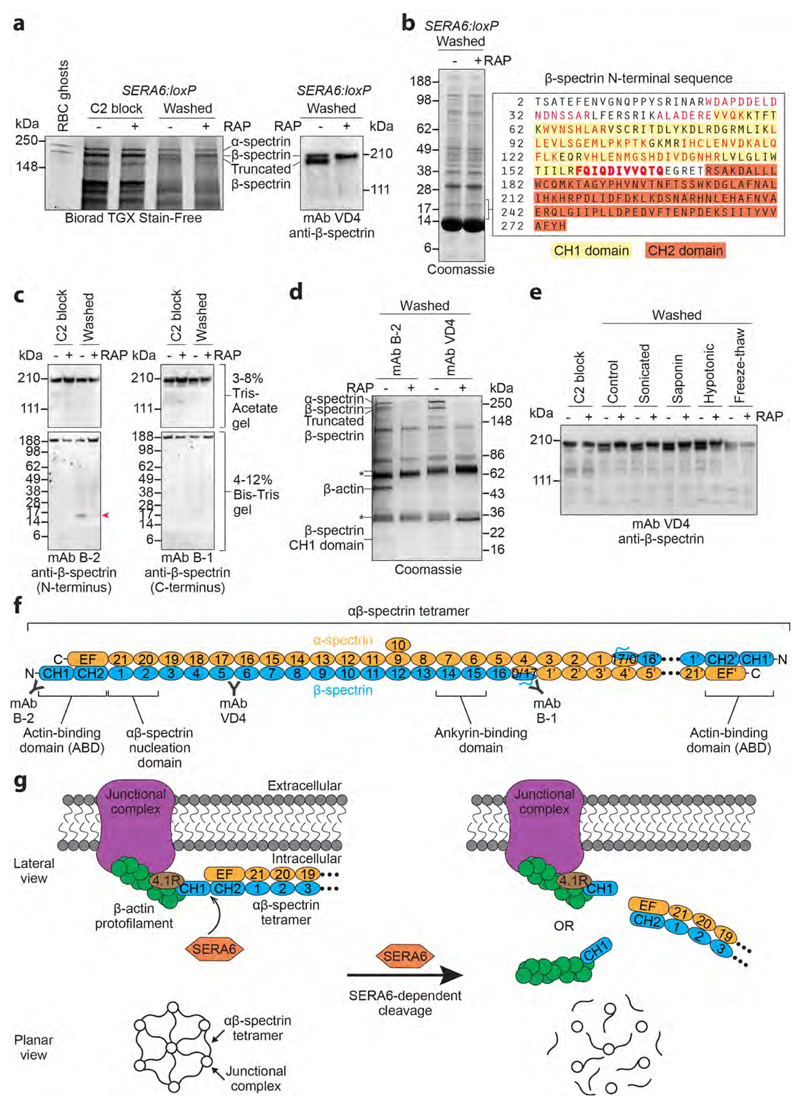
Figure 4. RBCM rupture is associated with rapid, SERA6-dependent cleavage of host RBC cytoskeleton β-spectrin within its actin-binding domain.
a, SDS PAGE showing appearance upon egress of mock-treated (-RAP) SERA6:loxP schizonts of a high molecular mass species identified by Western blot and LC-MS/MS as truncated β-spectrin (reproducible in 15 independent experiments). b, Peptides (red) identified by LC-MS/MS (3 technical replicate runs from a single biological experiment) of tryptic digests of polypeptide(s) enriched in the mock-treated SERA6:loxP schizont extract in the indicated region of the gel (˜15-20 kDa), indicating egress-associated, SERA6-dependent cleavage of β-spectrin. A semi-tryptic peptide likely representing the C-terminus of the polypeptide(s) is in bold (Supplementary Fig. 6 shows fragmentation spectra). Calculated mass of the β-spectrin sequence (UniProtKB P11277) from Thr2-Gln167 is 19,251 Da. CH1, CH2, calponin homology domains. c, Appearance of a ˜17 kDa N-terminal fragment of β-spectrin (arrowed) upon egress of mock-treated SERA6:loxP schizonts (reproducible in 4 independent experiments). d, Pull-down of cytoskeletal components from soluble extracts of egressing SERA6:loxP schizonts. Annotated species, including co-precipitating β-actin, were identified by LC-MS/MS or Western blot (reproducible in 3 independent experiments). Peptide fingerprinting of the ˜17 kDa β-spectrin CH1 domain was as in b. The presence of α-spectrin and full-length and truncated β-spectrin in pull-downs from the -RAP extracts indicates their SERA6-dependent dissociation from the normally insoluble cytoskeleton. Antibody heavy and light chains, asterisked. e, Fate of β-spectrin in SERA6:loxP schizonts following washing away a C2 block (control) or with additional treatment by the indicated disruption methods. Cleavage never occurred in the absence of SERA6 (Western blot representative of 2 independent experiments). f, Architecture of RBC cytoskeleton spectrin heterotetramer, comprising 2 antiparallel αβ-spectrin heterodimers linked head-to-head (the right-hand dimer is abbreviated for clarity) which cross-link β-actin-containing junctional complexes24. Spectrin repeat domains are numbered. Other structural features and positions of epitopes recognised by mAbs B-1, B-2 and VD4 are indicated. g, Top, SERA6-dependent cleavage of β-spectrin should release each end of the αβ-spectrin tetramer from its cognate junctional complex. The cleaved CH1 domain may be released with actin still bound. Bottom, predicted global effect of SERA6-dependent cleavage on the cytoskeleton.