Abstract
BACKGROUND
The optimal immunosuppressive regimen in kidney transplant recipients, delivering maximum efficacy with minimal toxicity, is unknown.
METHODS
The Amsterdam, LEiden, GROningen trial is a randomized, multicenter, investigator-driven, noninferiority, open-label trial in 305 kidney transplant recipients, in which 2 immunosuppression minimization strategies—one consisting of early steroid withdrawal, the other of tacrolimus minimization 6 months after transplantation—were compared with standard immunosuppression with basiliximab, corticosteroids, tacrolimus, and mycophenolic acid. The primary endpoint was kidney function. Secondary endpoints included death, primary nonfunction, graft failure, rejection, discontinuation of study medication, and a combined endpoint of treatment failure. An interim analysis was scheduled at 6 months, that is, just before tacrolimus minimization.
RESULTS
This interim analysis revealed no significant differences in Modification of Diet in Renal Disease between the early steroid withdrawal group and the standard immunosuppression groups (43.2 mL/min per 1.73 m2 vs 45.0 mL/min per 1.73 m2, P = 0.408). There were also no significant differences in the secondary endpoints of death (1.0% vs 1.5%; P = 0.737), primary nonfunction (4.1% vs 1.5%, P = 0.159), graft failure (3.1% vs 1.5%, P = 0.370), rejection (18.6% vs 13.6%, P = 0.289), and discontinuation of study medication (19.6% vs 12.6%, P = 0.348). Treatment failure, defined as a composite endpoint of these individual secondary endpoints, was more common in the early steroid withdrawal group (P = 0.027), but this group had fewer serious adverse events and a more favorable cardiovascular risk profile.
CONCLUSIONS
Based on these interim results, early steroid withdrawal is a safe short-term immunosuppressive strategy. Long-term outcomes, including a comparison with tacrolimus minimization after 6 months, will be reported in the final 2-year analysis.
Immunosuppression with basiliximab, prednisolone, calcineurin inhibitors, and mycophenolic acid results in low rejection rates and excellent graft survival in kidney transplant recipients.1-7 Despite this success, mortality and morbidity rates remain relatively high due to infectious complications, malignancies, an increased cardiovascular risk, and other long-term side effects of immunosuppression.
The Amsterdam, LEiden, GROningen (ALLEGRO) trial was designed to compare 2 immunosuppression minimization strategies—early steroid withdrawal and tacrolimus minimization after 6 months—to standard immunosuppression with basiliximab, corticosteroids, tacrolimus, and mycophenolic acid. The aim of the study is to assess whether early steroid withdrawal or tacrolimus minimization can provide equivalent outcomes in terms of kidney function while limiting immunosuppressive toxicity. In this interim analysis, the 6-month results of early steroid withdrawal are compared to those of standard immunosuppression. The final analysis will report the 2-year outcomes and will also include the results of the tacrolimus minimization group.
MATERIALS AND METHODS
Study design
We conducted a prospective, open label, multicenter, randomized, investigator-driven trial comparing standard immunosuppression (basiliximab/corticosteroids/tacrolimus/mycophenolic acid) to early steroid withdrawal and to tacrolimus minimization after 6 months.
In this trial, kidney transplant recipients from 3 participating Dutch academic medical centers were included: the Academic Medical Center in Amsterdam, Leiden University Medical Center, and the University Medical Center Groningen. Approval from the Institutional Review Board of the participating institutions was obtained, and the trial was conducted in compliance with the principles of Good Clinical Practice, the Declaration of Helsinki, and national laws and regulations. All patients provided written informed consent and could withdraw from the study at any time.
Patients between the ages of 18 and 80 years who were scheduled to receive a first or second kidney transplant from a living donor, donation after brain death (DBD) donor, or donation after cardiac death (DCD) donor were eligible to participate in this trial. Patients receiving a kidney from an HLA-identical related donor were excluded, as were patients who had more than 75% current or historic panel reactive antibodies, patients with diabetes mellitus type I, and female patients who were unwilling to use adequate contraception during the study.
Before undergoing kidney transplantation, patients were randomly assigned in a 1:1:1 ratio to 3 treatment groups (groups 1, 2a, and 2b) by means of a centralized, interactive voice-response system. Randomization did not take into account any specific patient or donor organ characteristic, such as organ type. All groups received induction therapy with basiliximab and methylprednisolone. Group 1, the early steroid withdrawal group, received no prednisolone maintenance immunosuppression. Groups 2a and group 2b both received standard prednisolone, tacrolimus, and mycophenolic acid for the first 6 months. After 6 months, group 2b was switched to a low-dose tacrolimus regimen for the remainder of the study. The total study duration was set at 2 years, with a prespecified interim analysis scheduled 6 months after the last patient had been included. Figure 1 provides a schematic overview of the study.
FIGURE 1.

Schematic overview of the study.
Detailed Study Medication
All groups received induction treatment with basiliximab (Simulect, 20 mg intravenously on day 0 and day 4) and methylprednisolone (500 mg, 250 mg, 125 mg intravenously on days 0, 1, and 2). Mycophenolic acid (MyFortic) was prescribed at 720 mg twice daily for the first 2 weeks and then tapered to 540 mg twice daily for the remainder of the study. Group 1 received no maintenance prednisolone, whereas prednisolone in groups 2a and 2b was dosed at 10 mg once daily for the first 6 weeks and then lowered to 7.5 mg once daily for the remainder of the study.
All subjects were given extended-release tacrolimus (Advagraf), with a trough level target of 8 to 12 ng/mL for the first 6 weeks, which was then lowered to 6 to 10 ng/mL. For group 1 and 2a, this target trough level was continued for the remainder of the study, whereas in group 2b (the tacrolimus minimization group), target trough levels were lowered to 3 to 5 ng/mL after 6 months.
Patients with evidence of either donor or recipient cytomegalovirus seropositivity received 6 months of valgancyclovir (Valcyte) prophylaxis. In addition, all patients were prescribed 6 months of Pneumocystis jirovecii prophylaxis (trimethoprim/sulfamethoxazole or Cotrimoxazole).
Safety
All adverse events were monitored and recorded. A data safety monitoring board was formed, which met after 75 and 150 patients had been included to judge the rate of rejections and serious adverse events (SAEs). The data safety monitoring board had the right to terminate the study if the rejection rate was higher than 30%.
Rejection
Indication biopsies were performed at the discretion of the treating physician. Rejections were treated identically in both groups, according to local practice. Patients in the early steroid withdrawal group with a documented rejection were switched to standard maintenance immunosuppression. Protocol biopsies in both groups were performed at 1 and 2 years after kidney transplantation and are therefore not included in this 6-month interim analysis.
Efficacy
The primary endpoint of this study was kidney function, measured as estimated glomerular filtration rate (eGFR) by means of the Modification of Diet in Renal Disease (MDRD) equation. In addition, creatinine clearance and proteinuria were obtained from 24-hour urine collections.
Secondary endpoints for the interim analysis consisted of graft and patient survival, documented rejection episodes, interruption of study medication for more than 6 weeks, SAEs and cardiovascular risk factors (blood pressure, lipid profile, and diabetes). In addition, a composite endpoint reflecting treatment failure was defined as death, primary nonfunction, graft failure (ie, death-censored graft loss), a documented rejection, or interruption of study medication for more than 6 weeks. If patients experienced multiple events, only their first event was included in the composite endpoint of treatment failure.
Statistical Methods
The sample size was calculated assuming 80% power to detect noninferiority in terms of eGFR at a significance level of 5%. Noninferiority was defined as a difference of 10 mL/min per 1.73 m2 or less in mean eGFR. The standard deviation of eGFR was estimated at 25 mL/min per 1.73 m2. This implies a group size of at least 75 patients for each of the 3 groups included in the final analysis. Analyses were performed both on an intention-to-treat and as-treated basis.
For the interim analysis, Student t tests (for MDRD and creatinine clearance) and Mann-Whitney U tests (for proteinuria) were used depending on the characteristics of the underlying distribution. In case of graft failure, an eGFR of 10 mL/min per 1.73 m2 and a creatinine clearance of 10 mL/min were imputed. Sensitivity analyses were run both with and without imputations for graft failure. Secondary endpoints were compared by Kaplan-Meier analyses (for death, primary nonfunction, graft failure, rejection, interruption of study medication and the composite endpoint of treatment failure), χ2 tests (for SAEs), and analysis of covariance analyses (for cardiovascular risk factors).
RESULTS
Patients
From June 27, 2011, to August 6, 2014, 305 patients underwent randomization (Figure 2). Eight patients had an inclusion/exclusion violation, so that 297 patients could be included in the intention-to-treat analysis. After 6 months, 53 of 98 patients had completed the assigned treatment in the early steroid withdrawal group, compared with 133 of 199 patients in the standard immunosuppression group.
FIGURE 2.

Enrollment and outcomes.
The baseline characteristics of patients in the early steroid withdrawal and standard immunosuppression group are described in Table 1. It shows that both groups were well balanced in terms of demographic characteristics, underlying renal disease, previous renal replacement therapy, comorbidity, and donor and surgical characteristics.
TABLE 1.
Baseline characteristics

Tacrolimus trough levels were within predefined boundaries and were identical for all groups, with the exception of week 2 trough levels, which were slightly but significantly higher in the early steroid withdrawal group (Figure 3). From the third week onward, dosages were adequately adjusted so that trough levels between the groups were indistinguishable. Average mycophenolic acid dosages were not significantly different for both groups: 930 mg daily in the early steroid withdrawal group, compared to 994 mg daily in the standard immunosuppression group (P = 0.123).
FIGURE 3.

Tacrolimus trough levels.
Primary Endpoint
There were no statistically significant differences in kidney function between the 2 groups. This was true for MDRD, creatinine clearance, and proteinuria (Table 2), both for the intention-to-treat and the as-treated analysis. Sensitivity analyses were performed with and without imputations for graft failure, without significant changes in outcome.
TABLE 2.
Primary endpoint (kidney function)

Secondary Endpoints
Four patients died during the first 6 months of the ALLEGRO trial, 1 (1.0%) in the early steroid withdrawal group and 3 (1.5%) patients in standard immunosuppression group. The cause of death of the patient in the early steroid withdrawal group was pneumosepsis; in the standard immunosuppression group, the causes of death were pneumosepsis and 2 cases of sudden death at home. Primary nonfunction occurred in 4.1% of patients in the early steroid withdrawal group and in 1.5% of patients in the standard immunosuppression group, whereas graft failure occurred in 3.1% and 1.5% of patients, respectively. The combined rate of death, primary nonfunction, and graft failure was not significantly different between the 2 groups (P = 0.325, Figure 4A). Rejection (Table 3) occurred in 18.6% of patients in the early steroid withdrawal group compared with 13.6% in the standard immunosuppression group (P = 0.289, Figure 4B). There was no statistically significant difference in steroid-resistant rejections (P = 0.564). In the early steroid withdrawal group, 19.6% of patients had to discontinue their study medication, compared with 12.6% in the standard immunosuppression group (P = 0.164, Figure 4C). The reasons for the discontinuation of study medication were varied (see Table 4) and included infectious complications (4.1% in both groups), mycophenolic acid toxicity (3.1% in the early steroid withdrawal group vs 1.0% in the standard immunosuppression group, P = 0.382), and a requirement for prednisolone for reasons other than rejection (in 4.1% of patients in the early steroid withdrawal group, eg, due to interstitial nephritis or hyponatremia). Treatment failure, defined as the composite endpoint of death, primary nonfunction, graft failure, rejection, and interruption of study medication for more than 6 weeks, occurred more frequently in the early steroid withdrawal group (P = 0.024, Figure 4D).
FIGURE 4.
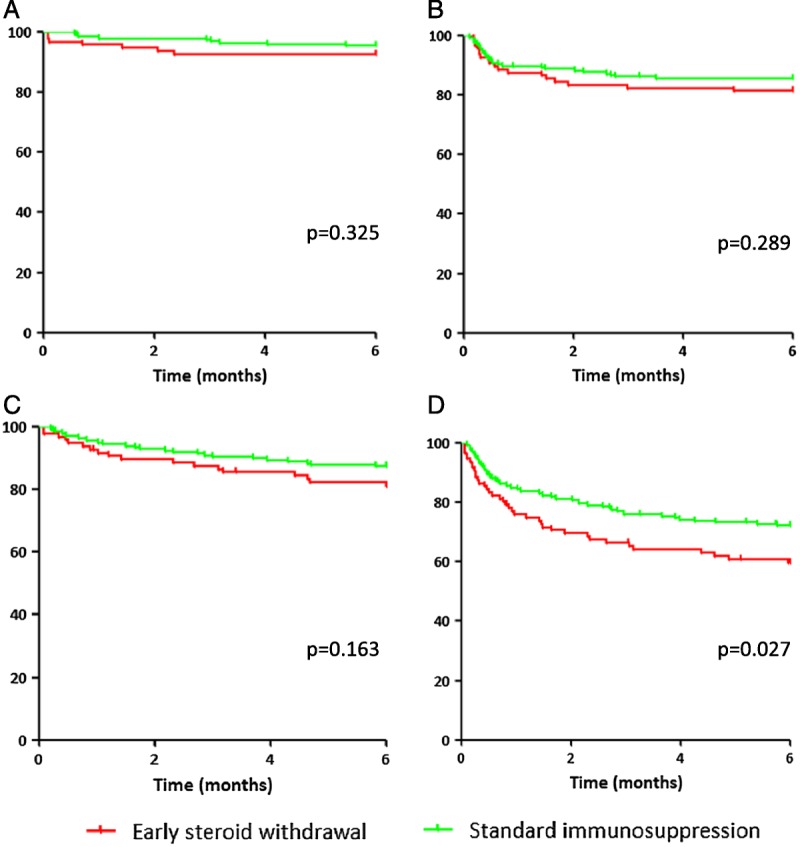
Percentage of patients free from (A) death, primary nonfunction, and graft failure, (B) rejection, (C) interruption of study medication, and (D) any type of treatment failure.
TABLE 3.
Rejections

TABLE 4.
Reasons for Discontinuation of study medication

Serious adverse events were less common in the early steroid withdrawal group (44.3 vs 56.6 per 100 patients, P = 0.048, Table 5), which was mostly attributable to a lower rate of infections. The early steroid withdrawal group also demonstrated a more favorable cardiovascular risk profile (Table 6): an improved diastolic blood pressure, and a lower total cholesterol and LDL, despite a lower percentage of patients on cholesterol lowering agents in the early steroid withdrawal group (32% vs 37.9%). The percentage of patients with new onset diabetes mellitus type II, defined as initiation of oral hypoglycemic agents or insulin for at least 30 consecutive days, was higher in the standard immunosuppression group (34.7% vs 24.3%), but due to a baseline difference in diabetes mellitus type 2 prevalence in both groups (24.2% in the standard immunosuppression group vs 14.4% in the early steroid withdrawal group), the increase was not statistically significant.
TABLE 5.
SAEs per 100 patients

TABLE 6.
Cardiovascular risk factors

Subgroup analysis for different donor types
No differences in MDRD were found for different donor subtypes, but living donor kidney recipients had a higher creatinine clearance (64 mL/min) and a lower median proteinuria (0.16 g/d) compared to DBD (53 mL/min; 0.19 g/d; P = 0.012) and DCD recipients (55 mL/min; 0.20 g/d; P = 0.047). These trends were the same for both the early steroid withdrawal and the standard immunosuppression group. Three of 4 deaths occurred in the DCD group and 1 in a living donor kidney recipient (P = 0.242). No significant differences in primary nonfunction and graft failure were found for different donor types, but there was a trend of a lower rejection rate in the DBD group (8% versus 19% in living donor kidney transplant recipients and versus 16% in DCD recipients, P = 0.117). For all donor types, there were no significant differences in the above outcomes between the early steroid withdrawal and the standard immunosuppression group.
DISCUSSION
These interim results of the ALLEGRO trial show that early steroid withdrawal in living donor, DBD, and DCD kidney transplant recipients is noninferior compared with standard maintenance immunosuppression with basiliximab, tacrolimus, mycophenolic acid, and corticosteroids in terms of kidney function at 6 months. Early steroid withdrawal has been evaluated in 2 recent meta-analyses,9,10 but the trials included in these meta-analyses were very heterogeneous in terms of timing of steroid withdrawal and concurrent immunosuppression. Only 4 trials are directly comparable to the ALLEGRO trial.11-14 These trials are summarized in Table 7 and confirm the noninferiority of early steroid withdrawal in terms of kidney function. What our analysis adds to these results is that early steroid withdrawal is also noninferior in terms of proteinuria, an important predictor of graft survival.15 We are also the first study to have included recipients of living donor, DBD, and DCD kidneys with a low to intermediate immunological risk.
TABLE 7.
Summary of studies investigating maintenance immunosuppression with tacrolimus and mycophenolic acid with or without steroids

In our study, early steroid withdrawal did not increase the 6-month incidence of the individual secondary endpoints of death, primary nonfunction, and graft failure. We did find a somewhat higher rate of rejection in the early steroid withdrawal group (18.6% vs 13.6%), but this difference was not statistically significant. This contrasts with the study by Woodle et al,13 who found a significantly higher rate of biopsy-proven rejection in the steroid withdrawal group (17.8% vs 10.8%), despite using either thymoglobulin, daclizumab, or basiliximab according to local center preference, whereas we used basiliximab in all cases. However, our study could be underpowered to detect differences in the rate of rejection.
Although rejection rates and patient and graft survivals were comparable in both groups, the early steroid withdrawal group was at increased risk for the composite endpoint of treatment failure. This was largely due to a higher percentage of discontinuation of study medication, for example, because of mycophenolic acid toxicity or a requirement for prednisolone for reasons other than rejection. The relatively high rate of discontinuation of study medication also explains the relatively small number of patients in our as-treated analysis, which is one of the limitations of our study. Other limitations include a relatively short follow-up duration and a heterogeneous (but real-life) study population. Lastly, the ALLEGRO study is not double-blind, but we believe that any bias would be limited, because tacrolimus levels were very comparable in both groups. The reported difference in tacrolimus levels in week 2 was most likely due to an interaction of prednisolone with tacrolimus, resulting in lower tacrolimus trough levels in the standard immunosuppression group. This phenomenon has been described previously16 and was confirmed by an analysis of average Advagraf dosages, which were slightly higher in the standard immunosuppression group (13.5 mg vs 12.7 mg once daily) despite lower trough levels in that group.
Based on these interim results, we believe that steroid-free maintenance immunosuppression is a safe short-term strategy for living donor, DBD and DCD kidney transplant recipients with a low to intermediate immunological risk. Although associated with an increased risk of treatment failure, it does not impair kidney function at 6 months, as well as having the benefits of a decreased risk of infections and an improved cardiovascular risk profile. Whether this will outweigh possible long-term risks, such as the possibility of increased donor-specific antibody formation or increased calcineurin inhibitor toxicity compared to the tacrolimus minimization group, will be addressed in our definitive 2-year analysis.
ACKNOWLEDGMENTS
The authors would like to thank GN Nieuwenhuizen, TSM Standaar, I Bunck, GPA Clerx and TC Timmer (Renal Transplant Unit, AMC), SAM van Berkel and S Hendriksen (Clinical Research Unit, Department of Medicine, LUMC), M van Dijk and AW Gomes Neto (Department of Nephrology, UMCG), S Boontje, B Scheerder and H Strooisma (Trial Coordination Center, UMCG) for their great contribution in collecting the data. The authors would also like to thank RJ Hene, RT Gansevoort and E de Maar for their participation in the DSMB.
Footnotes
Published online 15 May, 2018.
CLINICALTRIALS.GOV IDENTIFIER: NCT01560572
F.J.B. and J.-S.S.S. contributed equally to this work.
Astellas Pharma and Novartis provided financial support for the trial, but were not involved in the protocol design, data acquisition, analysis or reporting of the results.
The authors declare no conflict of interest.
M.S.v.S. wrote the initial draft of the article. A.P.J.dV., F.J.B., and J.S.S. designed the trial. These and all other authors reviewed the draft of the article, provided expertise for revisions, and approved the final version of the article.
REFERENCES
- 1.van Sandwijk MS, Bemelman FJ, Ten Berge IJ. Immunosuppressive drugs after solid organ transplantation. Neth J Med. 2013;71:281–9. [PubMed] [Google Scholar]
- 2.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29. [DOI] [PubMed] [Google Scholar]
- 3.Webster A, Woodroffe RC, Taylor RS, et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005;4:CD003961. [DOI] [PubMed] [Google Scholar]
- 4.Remuzzi G, Cravedi P, Constantini M, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow-up randomized, controlled clinical trial. J Am Soc Nephrol. 2007;18:1973–85. [DOI] [PubMed] [Google Scholar]
- 5.Ekberg H, Tedesco-Silva H, Demirbas A, et al. ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–75. [DOI] [PubMed] [Google Scholar]
- 6.Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–43. [DOI] [PubMed] [Google Scholar]
- 7.Guerra G, Ciancio G, Gaynor JJ, et al. Randomized trial of immunosuppressive regimens in renal transplantation. J Am Soc Nephrol. 2011;22 (9):1758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual J, Royuela A, Galeano C, et al. Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrol Dial Transplant. 2012;27:825–32. [DOI] [PubMed] [Google Scholar]
- 10.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1–14. [DOI] [PubMed] [Google Scholar]
- 11.Laftavi MR, Stephan R, Stefanick B, et al. Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery. 2005;137:364–71. [DOI] [PubMed] [Google Scholar]
- 12.Rostaing L, Cantarovich D, Mourad G, et al. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation. 2005;79:807–14. [DOI] [PubMed] [Google Scholar]
- 13.Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564–77. [DOI] [PubMed] [Google Scholar]
- 14.Kramer BK, Klinger M, Vitko S, et al. Tacrolimus-based, steroid-free regimens in renal transplantation: 3-year follow-up of the ATLAS trial. Transplantation. 2012;94:492–8. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Fresnedo G, Plaza JJ, Sánchez-Plumed J, et al. Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant. 2004;Suppl 3:iii47–iii51. [DOI] [PubMed] [Google Scholar]
- 16.Shihab FS, Lee ST, Smith LD, et al. Effect of corticosteroid withdrawal on tacrolimus and mycophenolate mofetil exposure in a randomized multicenter study. Am J Transplant. 2013;13:474–84. [DOI] [PubMed] [Google Scholar]


