Fig. 1 |. Classical and newly discovered DC subsets.
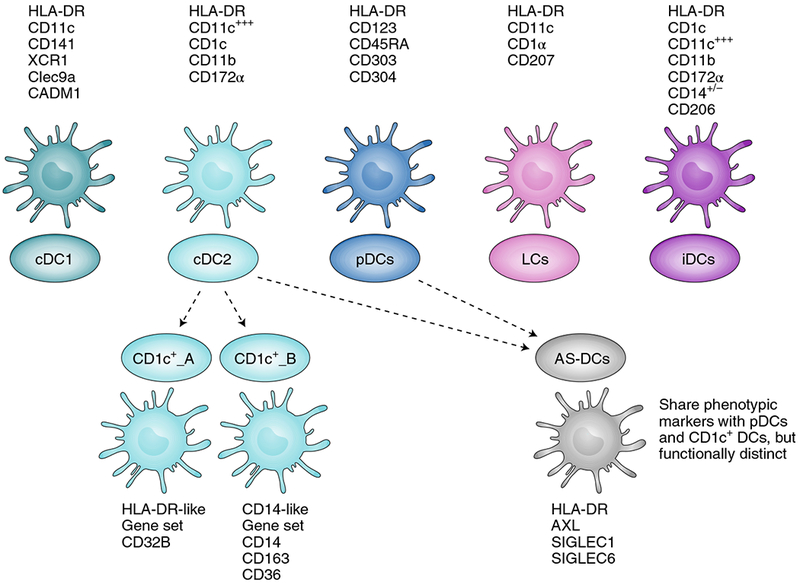
DCs have been divided into several subsets, namely conventional DCs (cDC1, cDC2), plasmacytoid DCs (pDCs), Langerhan cells (LCs) and inflammatory DCs (iDCs), according to their functional properties and phenotype. Cell surface markers generally used to identify these subsets from blood and tissue are listed with each cell type. cDC1 and cDC2 can present antigens on MHC-I and MHC-II, and can thus activate both CD8+ and CD4+ T cells. cDC1 are primarily lymph-node-resident DCs recognized for their exceptional capacity for antigen crosspresentation and for activating CD8+ T cells. They can secrete high levels of type-I and type-III IFNs and IL-12 when stimulated with double-stranded RNA (dsRNA) poly-IC:LC (poly-inosinic and poly-cytidylic acids stabilized with poly-lysine). cDC2 are migratory DCs known for activating CD4+ T cells and for secreting high levels of IL-12 on activation. pDCs are primarily type-I IFN-secreting cells, and play a role in activating other DC subsets, T cells and B cells. Single-cell sequencing and unbiased genome classification of HLA-DR+Lin− cells isolated from blood suggests the existence of additional DC subsets (dotted lines), namely CD1c+_A, CD1c+_B and Axl+SIGLEC6+ DCs (AS-DCs). CD1c+_A and CD1c+_B are new subsets found within conventional cDC2 (CD1c+). The newly described AS-DC subset bears phenotypic markers similar to those found in both pDC and CD1c subsets, but deeper analysis revealed that AS-DCs were functionally distinct from pDCs despite their phenotypic similarity6.
