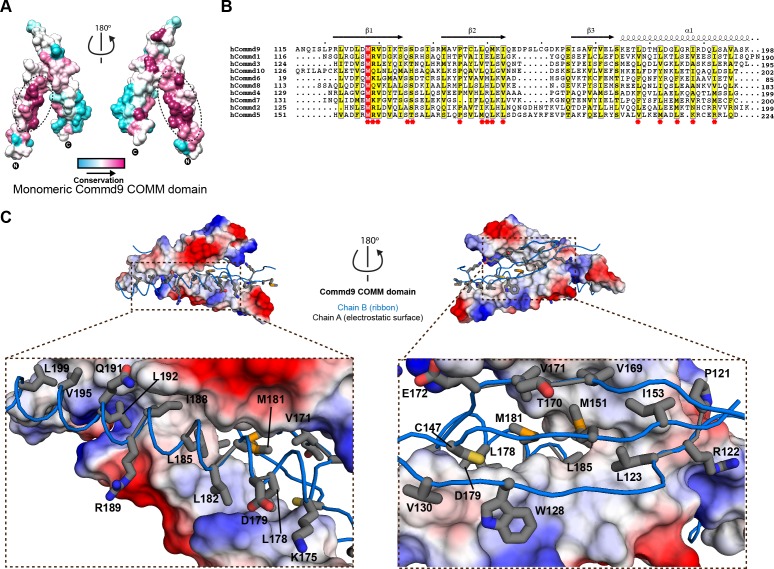
Figure 2. Structural mechanism underpinning the COMMD dimerization.
(A) A surface map of the conserved and variable residues of the Commd9 COMM domain showing the hydrophobic core is highly conserved while the surface residues are more variable, confirming the importance of dimerization for COMMD stability. These calculations are made using the Consurf server (Ashkenazy et al., 2010) on chain A. (B) A combined sequence alignment and secondary structure comparison of COMM domains of all the human COMMD proteins highlights that the C-terminal COMM domain is highly conserved across the COMMD family of proteins. Residues marked by asterisk depict conservation of amino acids that decorate the dimerization interface. Alignments were made with ESPript 2.2 (http://espript.ibcp.fr/ESPript/ESPript/) (Gouet et al., 2003) (C) Representation of dimer interface of Commd9 COMM domain highlighting key residues (chain A, worm in blue) making main chain-main chain, stacking, salt bridge interactions with the electrostatic surface (chain B).