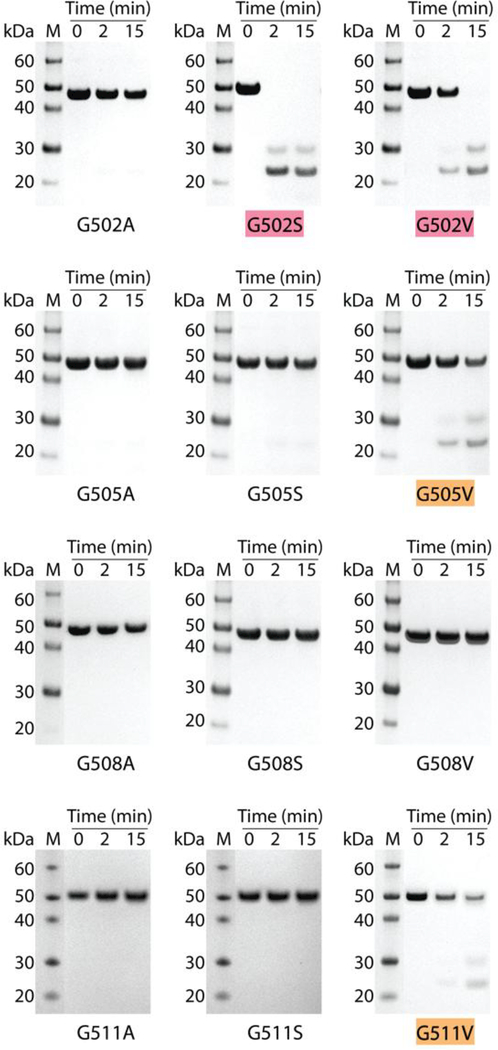
Figure 3.
Trypsin digestion of the recombinant bacterial protein with Gly to Ala, Gly to Ser and Gly to Val mutations at positions 502, 505, 508 and 511 in the 6-triplet insertion. SDS- PAGE of all recombinant collagens after trypsin digestion for time t=0, 2, and 15 min at 20°C. G502S, G502V, G505V and G511V showed various levels of trypsin susceptibility. Mutants completely degraded within 15 min were highlighted with red; mutants susceptible to trypsin but incompletely degraded within 15 min were highlighted with orange. Lane M, Novex® Sharp protein standard (Invitrogen); collagen monomer chains run slower than expected, as reported previously; molecular mass indicated in kDa.