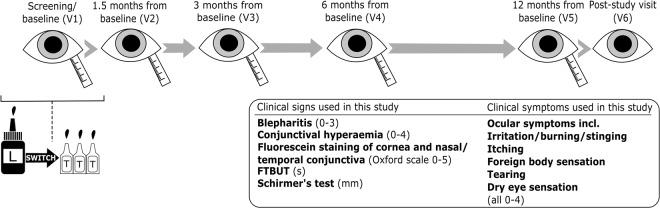
Figure 6.
Study outline summary. During the screening/baseline visit (V1), the patients were switched from preserved latanoprost (L) to preservative-free tafluprost (T) in unit dose dispensers. Clinical measurement together with the tear sample collection were performed at visits V1-V5. At the post-study visit (V6), final clinical measures were recorded but tear samples were no longer collected. FTBUT, fluorescein tear break-up time.