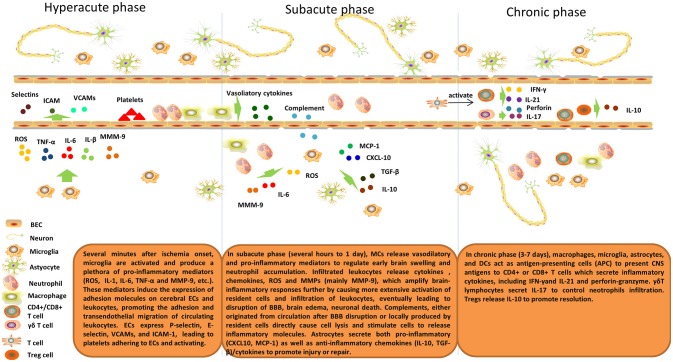
Figure 1.
Inflammatory response after cerebral ischemia. Brain post-ischemic inflammatory responses are characterized by innate immune activation followed by adaptive immune activation. Microglial cells are activated within minutes of ischemia onset and produce a plethora of pro-inflammatory mediators (ROS, IL-1, IL-6, TNF-α, and MMP-9, etc.). These mediators induce expression of adhesion molecules on cerebral ECs and leukocytes and, thus, promote adhesion and transendothelial migration of circulating leukocytes. ECs express P-selectin, E-selectin, VCAMs, and ICAM-1, which lead to platelets adhering to and activating ECs. In the subacute phase (hours to 1 day), MCs release vasodilatory and pro-inflammatory mediators to regulate early brain swelling and neutrophil accumulation. Infiltrating leukocytes release cytokines, chemokines, ROS and MMPs (mainly MMP-9), which amplify brain-inflammatory responses further by causing more extensive activation of resident cells and infiltration of leukocytes, eventually leading to disruption of the BBB, brain edema, and neuronal death. Complements, either originating from the circulation after BBB disruption or locally produced by resident cells directly cause cell lysis and stimulate cells to release inflammatory molecules. Astrocytes secrete both pro-inflammatory (CXCL10, MCP-1) and anti-inflammatory chemokines (IL-10, TGF-β)/cytokines to promote injury or repair. In the delayed phase (3–7 days), macrophages, microglia, astrocytes, and DCs act as antigen-presenting cells (APCs) to present CNS antigens to CD4+ or CD8+ T cells that secrete inflammatory cytokines, including IFN-γ and IL-21 and perforin-granzyme. γδT lymphocytes secrete IL-17 to control neutrophil infiltration. Tregs release IL-10 to promote resolution.