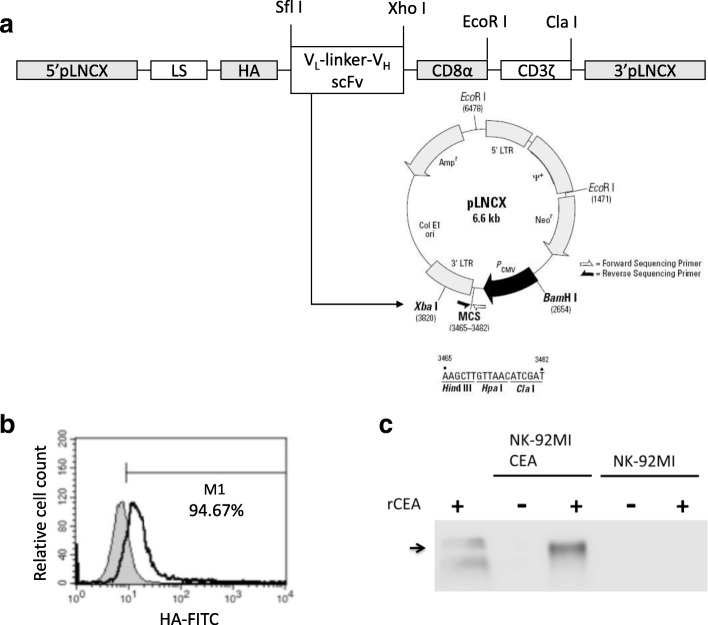
Fig. 1.
Genetic modification of NK-92MI cells with anti-CEA-CD8α-CD3ζ chimeric receptor. a Schematic image of the chimeric receptor anti-CEA-CD8α-CD3ζ. The chimeric receptor consisted of the VL and VH regions of the anti-CEA mAb joined to a CD8α and fused to the transmembrane and intracellular regions of human TCR-CD3ζ. Map of destination vector pLNCX wherein the cDNA for the fusion protein anti-CEA-CD8α-CD3ζ was cloned into SfiI and ClaI restriction enzyme sites of modified retroviral pLNCX vector containing leader sequence and HA tag and sequenced for identification. The product was pLNCX- anti-CEA scFv-CD8α-CD3ζ. Transfected cells expressing the transgene of interest were selected on cytocidal concentrations of neomycin sulphate (G418). b Surface expression of chimeric anti-CEA scFv-CD8α-CD3ζ. NK-92MI cells were analysed following staining with FITC-labelled HA tag Ab. Briefly, CAR expression was determined by flow cytometry with HA-tagged- and recognised anti-CEA chimeric receptor (green open area). Parental NK-92MI cells served as control (blue filled area). c Ability of anti-CEA chimeric receptor to recognise recombinant human CEA was determined by immunoblotting. Lysates of NK-92MI (lane 4) and transduced anti-CEA NK-92MI cells (lane 2) were separated by SDS-PAGE. Transduced anti-CEA NK-92MI or parental NK-92MI co-cultured with rCEA (lanes 3 and 5) were analysed by immunoblot analysis