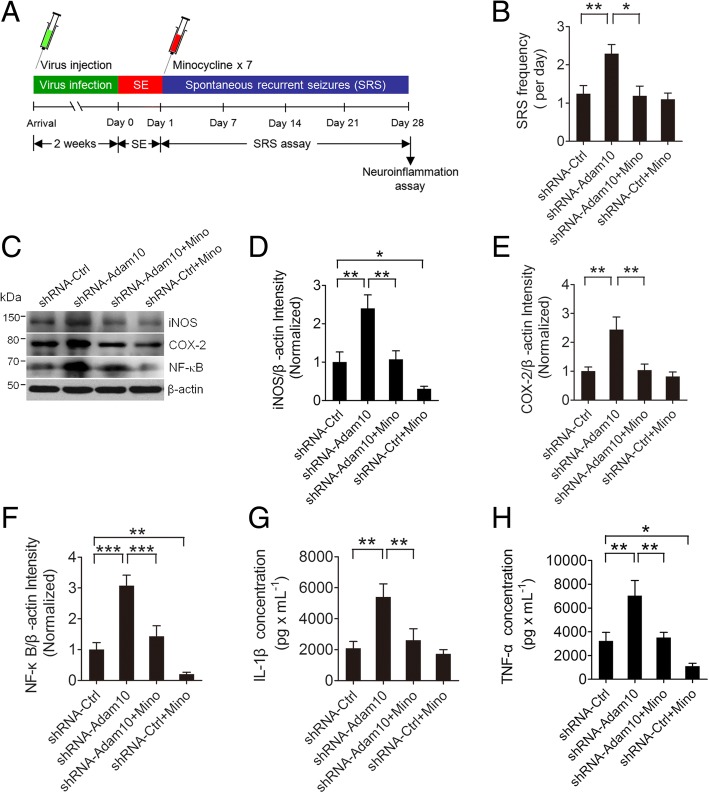
Fig. 9.
Increased seizure activity by Adam10 knockdown is dependent on hippocampal neuroinflammation. a Schematic diagram of the experimental design. Mice were bilaterally injected into the hippocampus with either Vehicle Ctrl or lentivirus carrying the shRNA-Ctrl or shRNA-Adam10. Following 2 weeks of recovery, the mice were induced to SE, and 24 hours after the SE induction, they were treated with minocycline (50 mg/kg, i.p.) seven times at 24-hour intervals. The mice were continuously video EEG monitored for 4 weeks for SRS analysis. The mice were then sacrificed after the EEG recording was completed at day 28 post-SE for analysis of hippocampal neuroinflammation. b Bar graph showing the SRS frequency in the shRNA-Ctrl, shRNA-Adam10, and shRNA-Adam10 + Minocycline- and shRNA-Ctrl + Minocycline-treated TLE mice. A two-way ANOVA revealed a significant main effect of Adam10 knockdown (F1,20 = 6.60, p = 0.02), minocycline treatment (F1,20 = 7.90, p = 0.011), and Adam10 knockdown × minocycline interaction (F1,20 = 4.69, p = 0.043) on SRS frequency. A Tukey post hoc test revealed that SRS frequency was significantly increased in shRNA-Adam10 mice compared to that in shRNA-Ctrl mice (p = 0.003). Minocycline treatment suppressed the shRNA-Adam10-induced increase of SRS frequency (p = 0.02), while the minocycline-treated shRNA-Ctrl mice did not show any significant difference of SRS frequency compared to the shRNA-Ctrl mice (p = 0.087) (n = 6). c Western blotting showing the protein levels of the inflammation-related proteins iNOS, COX-2, and NF-κB in the hippocampus of shRNA-Ctrl, shRNA-Adam10, and shRNA-Adam10 + Minocycline- and shRNA-Ctrl + Minocycline-treated TLE mice. d–f Bar graphs showing the quantification of iNOS, COX-2, and NF-κB as measured by the intensity ratios of these proteins to β-actin. For iNOS, a two-way ANOVA revealed a significant main effect of both Adam10 knockdown (F1,16 = 19.13, p < 0.001) and minocycline treatment (F1,16 = 16.67, p < 0.001) on iNOS protein level, but there was no significant interaction between Adam10 knockdown and minocycline treatment (F1,16 = 1.60, p = 0.224). A Tukey post hoc test revealed that the iNOS protein content was significantly increased in shRNA-Adam10 mice compared to that in shRNA-Ctrl mice (p = 0.001). Minocycline treatment suppressed the shRNA-Adam10-induced increase in iNOS protein level (p = 0.002), Moreover, the iNOS protein level in minocycline-treated shRNA-Ctrl mice was significantly decreased compared to that in shRNA-Ctrl mice (p = 0.044). For COX-2, a two-way ANOVA revealed a significant main effect of Adam10 knockdown (F1,16 = 9.98, p = 0.006), minocycline treatment (F1,16 = 9.05, p = 0.008), and Adam10 knockdown × minocycline interaction (F1,16 = 5.37, p = 0.034) on the COX-2 protein level. A Tukey post hoc test revealed that COX-2 protein content was significantly increased in shRNA-Adam10 mice compared to that in shRNA-Ctrl mice (p = 0.001). Minocycline treatment suppressed the shRNA-Adam10-induced increase in COX-2 protein level (p = 0.002), while the minocycline-treated shRNA-Ctrl mice did not show any significant difference in the COX-2 protein content compared to that in shRNA-Ctrl mice (p = 0.631). For NF-κB, a two-way ANOVA revealed a significant main effect of both Adam10 knockdown (F1,16 = 37.88, p < 0.001) and minocycline treatment (F1,16 = 20.67, p < 0.001) on the NF-κB protein level, but there was no significant interaction between Adam10 knockdown and minocycline treatment (F1,16 = 2.46, p = 0.136). A Tukey post hoc test revealed that the NF-κB protein content was significantly increased in shRNA-Adam10 mice compared to that in shRNA-Ctrl mice (p < 0.001). Minocycline treatment suppressed the shRNA-Adam10-induced increase in NF-κB protein level (p < 0.001). Moreover, the NF-κB protein level in minocycline-treated shRNA-Ctrl mice was significantly decreased compared to the levels in the shRNA-Ctrl mice (p = 0.008) (n = 5). g, h Bar graphs showing the concentration of IL-1β and TNF-α in the hippocampus of shRNA-Ctrl, shRNA-Adam10, and shRNA-Adam10 + Minocycline- and shRNA-Ctrl + Minocycline-treated TLE mice as detected by ELISA. For IL-1β, a two-way ANOVA revealed a significant main effect of both Adam10 knockdown (F1,16 = 11.14, p = 0.004) and minocycline treatment (F1,16 = 6.31, p = 0.023) on IL-1β concentration, but there was no significant interaction between Adam10 knockdown and minocycline treatment (F1,16 = 3.781, p = 0.070). A Tukey post hoc test revealed that the IL-1β concentration was significantly increased in shRNA-Adam10 mice compared to that in shRNA-Ctrl mice (p = 0.002). Minocycline treatment suppressed the shRNA-Adam10-induced increase in IL-1β concentration (p = 0.006), while the minocycline-treated shRNA-Ctrl mice did not show any significant difference in IL-1β concentration compared to that in shRNA-Ctrl mice (p = 0.693). For TNF-α, a two-way ANOVA revealed a significant main effect of both Adam10 knockdown (F1,16 = 16.09, p = 0.001) and minocycline treatment (F1,16 = 13.32, p = 0.002) on TNF-α concentration, but there was no significant interaction between Adam10 knockdown and minocycline treatment (F1,16 = 0.83, p = 0.375). A Tukey post hoc test revealed that the TNF-α concentration was significantly increased in shRNA-Adam10 mice compared to that in shRNA-Ctrl mice (p = 0.003). Minocycline treatment suppressed the shRNA-Adam10-induced increase in TNF-α concentration (p = 0.005). Moreover, the TNF-α concentration in minocycline-treated shRNA-Ctrl mice was significantly decreased compared to that in shRNA-Ctrl mice (p = 0.041) (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001, and two-way ANOVA