Abstract
The medical records of 87 dogs treated with surgery for cutaneous malignant melanoma (CMM) of the haired skin were retrospectively reviewed for overall survival time (OST), progression-free survival time (PFS), and prognostic factors. The post-surgery median PFS and median OST were 1282 days and 1363 days, respectively. The post-surgery metastatic rate was 21.8% with a local recurrence rate of 8%. Increasing mitotic index (MI) was predictive of a significantly decreased OST and PFS on multivariable analysis [hazard ratio (HR): 1.05, 95% confidence interval (CI): 1.02 to 1.07 and HR: 1.04, 95% CI: 1.02 to 1.06, respectively]. Increasing age was likewise predictive of a significantly decreased OST and PFS on multivariable analysis (HR: 1.39, 95% CI: 1.17 to 1.65 and HR: 1.33, 95% CI: 1.14 to 1.54, respectively). These results confirm clinical impressions that long survival times are likely in dogs diagnosed with malignant melanoma of the haired skin when treated with surgery alone.
Résumé
Résultat post-chirurgical et facteurs de pronostic pour les mélanomes malins canins de la peau poilue : 87 cas (2003–2015). Les dossiers médicaux de 87 chiens traités à l’aide d’une chirurgie pour le mélanome malin cutané (MMC) de la peau poilue ont été évalués rétrospectivement pour le temps de survie global (TSG), le temps de survie sans progression (TSSP) et les facteurs de pronostic. Le TSSP médian après la chirurgie et le TSG médian étaient de 1282 jours et de 1363 jours, respectivement. Le taux métastasique après la chirurgie était de 21,8 % avec un taux de récurrence local de 8 %. L’augmentation de l’indice mitotique (IM) était prédictive d’un TSG et d’un TSSP réduits à l’analyse multivariable (ratio de risque [RR] : 1,05, intervalle de confiance [IC] de 95 % : 1,02 à 1,07 et RR : 1,04, IC de 95 % : 1,02 à 1,06, respectivement). La progression de l’âge était aussi prédictive d’une réduction importante du TSG et du TSSP à l’analyse multivariable (RR : 1,39, IC de 95 % : 1,17 à 1,65 et RR : 1,33, IC de 95 % : 1,14 à 1,54, respectivement). Ces résultats confirment les impressions cliniques que des longs délais de survie sont probables chez les chiens diagnostiqués avec le mélanome malin de la peau poilue lorsqu’ils sont uniquement traités à l’aide d’une chirurgie.
(Traduit par Isabelle Vallières)
Introduction
Malignant melanoma is a common neoplasm in the dog, with the most commonly affected sites being the oral cavity, eye, nailbed, and skin (1,2). These sites show a relatively consistent behavior with melanoma of the oral cavity behaving, on average, most aggressively (high degree of local invasion and frequent distant metastasis). In comparison, cutaneous malignant melanoma (CMM) tends to behave less aggressively; however, a subset of cutaneous melanomas will display more aggressive local behavior and metastasize (2). Standard treatment for melanoma of all sites is local control with surgery and/or radiation therapy. Although commonly recommended, the effectiveness of adjuvant therapy in canine melanoma is an area of ongoing debate (1,3–5).
In a previous study examining a large set of melanocytic tumors (both benign and malignant masses) grouped by anatomic site, 39% of all cutaneous melanocytic tumors were diagnosed as histologically malignant by the reporting pathologist, although only 12% of these actually displayed malignant behavior (defined as local recurrence or metastasis) and only 7% of patients died of melanoma-related causes (6). This study identified a mathematical model using nuclear atypia as being most predictive of aggressive behavior. Other previously identified prognostic factors in malignant melanoma (of all sites) include mitotic index (MI) [defined as the number of mitotic figures per 10 high power field (hpf )], nuclear atypia, lesion size, inflammation, necrosis, and the presence of metastasis (2). The most common prognostic factor used in CMM is an MI of 3 or higher, which predicts decreased survival. This MI cutoff has been identified in 2 studies (7,8). Ki-67 proliferative index (≥ 15%) and increased nuclear survivin expression have also been identified as negative prognostic factors in CMM (8,9). In humans, a staging system is used to further predict the behavior of CMM. This staging system takes into account the thickness of the primary tumor, tumor ulceration, lymph node status, and distant metastasis (10). While the human staging system is generally prognostic, a degree of heterogeneity exists within stages such that other prognostic factors have been identified to supplement stage. These include anatomic site, histotype, presence of activating oncogene mutations (most notably in BRAF), and tumor infiltrating lymphocyte (TIL) scores.
Despite the previously mentioned prognostic factors in canine CMM, many veterinarians find the clinical behavior of most of these masses to be benign. In this study, we sought to examine the clinical prognostic factors in a large group of CMM treated with surgery to identify and update prognostic factors in this disease process.
Materials and methods
Patient selection
A comprehensive medical records search was performed for the years 2004 to 2014 at the Colorado State University Veterinary Teaching Hospital and 13 other academic and specialty referral hospitals. Cutaneous melanoma of the paw pad was excluded, as were any cases with the melanoma located at mucocutaneous junctions.
Medical records were evaluated and the following information was extracted: age, weight, gender, and breed of the dog along with number, size, and location of the lesions. Tumor-specific information extracted from the original histopathology report, when available, was MI and completeness of excision (margins). The MI was occasionally reported as number of mitotic figures per single hpf, in which instances it was converted to the number of mitoses per 10 hpf by multiplying by a factor of 10. Margins were considered close when reported directly in the original pathology report as close or when < 5 mm of normal tissue was present on the lateral edge of the specimen. Staging tests were performed at the discretion of the owner and clinician, and therefore this information was not available for all cases. When available, staging test results (thoracic radiographs, abdominal ultrasound, computed tomography scan, complete blood cell count, biochemical analysis, and lymph node cytology) were recorded. Adjuvant treatments were recorded when available. Outcome information, including local recurrence, distant metastasis and death, was obtained from the medical record or telephone interviews with the primary care veterinarian or owner when possible. For patients with multiple lesions, the highest MI, lesion measurement, and any incomplete or close margins were included in statistical analysis.
Statistics
Continuous data were expressed as median and range, and categorical data as frequencies and percentages. Progression-free survival (PFS) was defined as the time from the date of diagnosis to the date of local recurrence, development of metastasis, or death from any cause. Overall survival time (OST) was calculated from the date of diagnosis to the date of death. Unless otherwise known, dogs that died were considered to be dead either secondary to their treatment or as a result of their disease. Patients lost to follow-up or still alive at the time of statistical analysis were censored for OST. Patients lost to follow-up or that were alive with no evidence of disease recurrence or metastasis at the time of statistical analysis were censored in the PFS analysis.
Kaplan–Meier estimation was used to estimate and display PFS and OST. Variables examined for effects on OST and PFS were lesion size (longest diameter), age at the time of diagnosis, lesion location, MI, completeness of resection, and adjuvant treatment. Generated survival curves were compared using the log-rank (Mantel-Cox) test. P-values < 0.05 were considered significant. Mitotic index cut-offs for OST and PFS were reached by first comparing MI above and below the median and then evaluating natural breakpoints with increasing MI until the highest MI that showed a significant difference between the 2 groups was identified. Multivariate analysis was performed using forward stepwise Cox regression, incorporating variables reaching significance on univariate analysis. All statistical analyses were performed using commercial software packages (Prism v.7; GraphPad Software, La Jolla, California, USA; SPSS v. 25; IBM, Armonk, New York, USA).
Results
Patient population and therapy
The initial medical records search at the Colorado State University Teaching Hospital revealed 438 unique cases with a diagnosis of malignant melanoma of any site. After evaluation of all cases, 13 cases with a clear diagnosis of CMM based on the original pathology report and medical record were identified. Due to a low case number, additional cases were solicited and obtained from multiple outside institutions and private referral practices via a standardized case accrual form using the same inclusion criteria as outlined. An additional 74 cases were submitted for a total of 87 cases accrued.
Patient demographics are presented in Table 1. All 87 patients were treated with a minimum of surgical resection of the primary tumor. Forty-eight patients received adjuvant therapy. Of these, 34 patients received single agent vaccine therapy. The most common vaccine therapy administered to patients was single agent therapy with a commercially available human tyrosinase based DNA vaccine (Oncept; Boehringer Ingelheim Vetmedica, Duluth, Georgia, USA) (n = 33), but 1 patient received an investigative melanoma vaccine via a clinical trial. Two patients received vaccine therapy that was combined with carboplatin, while another patient received vaccine therapy combined with oral low-dose cyclophosphamide. Six patients received single agent carboplatin. One patient received carboplatin followed by toceranib phosphate (Palladia). Two patients received carboplatin combined with radiation therapy while 2 patients received radiation therapy alone. Thirty-seven patients did not receive any adjuvant therapy. Two patients had unknown adjuvant therapy status.
Table 1.
Patient and tumor characteristics.
| Median [range or frequency (%)] | |
|---|---|
| Age (y) | 9.4 (2 to 13.9) |
| Gender | |
| Intact female | 4 (4.6) |
| Spayed female | 41 (47.1) |
| Intact male | 8 (9.2) |
| Castrated male | 34 (39.1) |
| Weight (kg) | 28.8 (3.2 to 54.5) |
| Breed | |
| Mixed breed | 15 (17.2) |
| Golden retriever | 12 (13.8) |
| Labrador retriever | 12 (13.8) |
| Doberman pinscher | 5 (5.7) |
| Miniature schnauzer | 5 (5.7) |
| Vizsla | 5 (5.7) |
| Dachshund | 4 (4.6) |
| Airedale | 3 (3.4) |
| Boxer | 2 (2.3) |
| Cocker spaniel | 2 (2.3) |
| German shepherd | 2 (2.3) |
| Irish setter | 2 (2.3) |
| Rottweiler | 2 (2.3) |
| Yorkshire terrier | 2 (2.3) |
| Other (1 each) | 14 (16.1) |
| Mitotic index | 6 (0 to 75) |
| Lesion longest diameter | 1.5 (0.3 to 4.5) |
| Margins | |
| Complete | 46 (56.1) |
| Close | 24 (29.3) |
| Incomplete | 12 (14.6) |
| Location | |
| Extremity | 44 (50.6) |
| Head | 21 (24.1) |
| Trunk | 20 (23) |
| Multiple sites | 2 (2.3) |
Tumor characteristics
Tumor location was divided into 4 broad groups (head, extremities, trunk, and dogs with multiple masses). A dog with multiple masses had lesions present on a digit and at the base of the prepuce. Another dog with multiple lesions had a lesion on the muzzle and in the peri-orbital region. Pre-surgical lesion measurements were available for 61 patients with the median presurgical maximal diameter being 1.5 cm (range: 0.3 to 4.5 cm).
Margin evaluation was reported for 82 patients and MI was reported in 60 patients (Table 1). The MI was reported as the number of mitoses per 10 hpf in 47 cases and as the number of mitoses per single hpf in 13 cases. Twenty-seven cases had no information regarding MI in the histopathology report. Eightytwo patients had thoracic radiographs or CT-scan of the thorax as part of their initial staging while 42 had regional lymph node evaluation via either fine-needle aspirate (FNA) or biopsy as part of the initial staging. Four patients had regional lymph node metastasis confirmed by FNA or biopsy and 2 patients had pulmonary nodules suspected to be metastasis based on thoracic imaging. One patient had both confirmed regional lymph node metastasis and suspected pulmonary metastasis identified at the time of presentation. For all patients with regional lymph node evaluation, the metastatic rate was 5/42 (11.9%). Pulmonary metastasis was identified in 3/82 patients for which pre-operative thoracic staging was performed (3.7%).
Outcome
Of the 87 total study patients, 49 were censored from OST analysis for the following reasons: 29 were alive at the time of analysis and 20 were lost to follow-up. The median follow-up time in this group was 747 d (range: 227 to 3246 d). Of the 87 total study patients, 43 patients were censored for PFS analysis for the following reasons: 26 were alive at the time of analysis and 17 were lost to follow-up with no evidence of recurrence or metastasis. The median follow-up time in this group was 705 d (range: 297 to 3246 d).
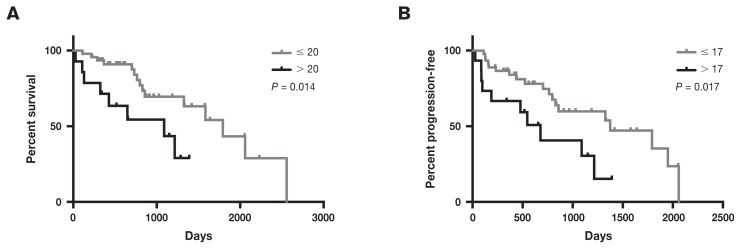
The median OST for the study population was 1363 d (range: 27 to 3246 d). Prognostic factors evaluated for OST are presented in Table 2. Two significant negative predictors of OST were identified, MI > 20 per 10 HPF (P = 0.014) and age > 9.4 y (the median age in the study) (P = 0.0001). The same prognostic factors were also analyzed as continuous variables for their effect on OST. Increasing age predicted a significantly decreased OST (HR: 1.291, 95% CI: 1.131 to 1.473) (P < 0.001). Increasing MI predicted a significantly decreased OST (HR: 1.023, 95% CI: 1.006 to 1.041) (P = 0.009). Completeness of margins, including those considered close, the presence of metastasis at the time of diagnosis, adjuvant therapy, and tumor location failed to predict OST in a univariate model.
Table 2.
Univariate analysis of prognostic factors for effect on overall survival in dogs with cutaneous malignant melanoma of the haired skin.
| Number of dogs | Median overall survival time (d) | Hazard ratio (95% confidence interval) | P-value | ||
|---|---|---|---|---|---|
| Mitotic index | |||||
| ≤ 20 | 46 | 1792 | |||
| > 20 | 14 | 1089 | 4.1 (1.3 to 12.9) | 0.014 | |
| Age (y) | |||||
| ≤ 9.4 | 44 | 1792 | |||
| > 9.4 | 43 | 797 | 3.7 (1.9 to 7.2) | 0.0001 | |
| Margins | |||||
| Complete | 46 | 1363 | |||
| Close | 24 | 1265 | 1.0 (0.5 to 2.1) | 0.991 | |
| Incomplete | 12 | not reached | 1.2 (0.4 to 3.7) | 0.759 | |
| Metastasis at diagnosis | |||||
| Pulmonary | |||||
| Negative | 79 | 1326 | |||
| Positive | 3 | not reached | 0.9 (0.1 to 6.3) | 0.935 | |
| Lymph node | |||||
| Negative | 37 | 1323 | |||
| Positive | 5 | 1792 | 1.4 (0.3 to 5.5) | 0.671 | |
| Adjuvant | |||||
| None | 37 | 1363 | |||
| Carboplatin | 11 | 2557 | 0.7 (0.3 to 1.9) | 0.504 | |
| Vaccine | 37 | 1282 | 0.8 (0.4 to 1.6) | 0.515 | |
| Size (cm) | |||||
| ≤ 1.5 | 35 | 1265 | |||
| > 1.5 | 26 | 1363 | 1.1 (0.5 to 2.5) | 0.835 | |
| Site | |||||
| Head | 21 | 2059 | |||
| Trunk | 20 | 1089 | 2.1 (0.8 to 5.3) | 0.110 | |
| Extremity | 44 | 1326 | 1.8 (0.8 to 4.4) | 0.176 | |
| Multiple sites | 2 | 441 | 3.4 (0.17 to 68.3) | 0.417 | |
The median PFS for the 87 patients included in the study was 1282 d (range: 27 to 3246 d). Twenty-four patients had confirmed or highly suspected progressive disease, including local recurrence (n = 5), development of CMM at distant cutaneous sites (n = 5), metastasis to regional lymph nodes (n = 4), new pulmonary nodules suspected to be metastasis (n = 2), confirmed metastasis to the spleen or liver (n = 2), new pulmonary nodules suspected to be metastasis and local recurrence (n = 2), new pulmonary nodules suspected to be metastasis and confirmed metastasis to the spleen or liver (n = 1), new pulmonary nodules suspected to be metastasis and confirmed metastasis to the regional lymph node (n = 1), suspected brain metastasis (n = 1), and metastasis to a location that was undefined in the medical record (n = 1). Based on this information, the calculated post-surgical metastatic rate in this study was 19/87 (21.8%). The local recurrence rate was 7/87 (8.0%). Prognostic factors evaluated for PFS are presented in Table 3. As with OST, increasing MI and increasing age were predictive of decreased PFS. The same prognostic factors were also analyzed as continuous variables for their effect on PFS. Increasing age predicted a significantly decreased PFS (HR: 1.431, 95% CI: 1.221 to 1.677) (P < 0.001). Increasing MI predicted a significantly decreased PFS (HR: 1.026, 95% CI: 1.007 to 1.046) (P = 0.008). There was no statistically significant difference in median PFS for adjuvant therapy, presence of metastasis at the time of diagnosis (to either the lungs or regional lymph nodes), margins, size, or tumor location.
Table 3.
Univariate analysis of prognostic factors for effect on progression-free survival in dogs with cutaneous malignant melanoma of the haired skin.
| Number of dogs | Median progression-free survival (d) | Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|---|---|
| Mitotic index | ||||
| ≤ 17 | 45 | 1374 | ||
| > 17 | 15 | 679 | 3.2 (1.2 to 8.6) | 0.017 |
| Age | ||||
| ≤ 9.4 y | 44 | 1792 | ||
| > 9.4 y | 43 | 764 | 2.7 (1.5 to 5.0) | 0.001 |
| Margins | ||||
| Complete | 46 | 1363 | ||
| Close | 24 | 857 | 1.8 (0.9 to 4.0) | 0.101 |
| Incomplete | 12 | 1092 | 1.5 (0.5 to 4.5) | 0.479 |
| Metastasis at diagnosis | ||||
| Thorax | ||||
| Negative | 79 | 1265 | ||
| Positive | 3 | not reached | 1.3 (0.2 to 7.3) | 0.765 |
| Lymph node | ||||
| Negative | 37 | 1484 | ||
| Positive | 5 | 1333 | 0.9 (0.3 to 3.4) | 0.922 |
| Adjuvant | ||||
| None | 37 | 1363 | ||
| Carboplatin | 11 | not reached | 1.6 (0.5 to 5.1) | 0.398 |
| Vaccine | 37 | 1089 | 1.2 (0.6 to 2.3) | 0.542 |
| Size | ||||
| ≤ 1.5 cm | 35 | 1265 | ||
| > 1.5 cm | 26 | 1326 | 1.1 (0.5 to 2.4) | 0.767 |
| Site | ||||
| Head | 21 | 1950 | ||
| Trunk | 20 | 1089 | 2.1 (0.9 to 5.3) | 0.104 |
| Extremity | 44 | 1265 | 1.9 (0.8 to 4.0) | 0.123 |
| Multiple sites | 2 | 177 | 9.0 (0.3 to 315.8) | 0.224 |
Upon multivariable analysis, both MI and age retained significance for PFS and OST (Table 4).
Table 4.
Multivariable analysis of prognostic factors for effect on progression-free survival and overall survival time in dogs with cutaneous malignant melanoma of the haired skin.
| Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|
| Progression-free survival | ||
| Mitotic index | 1.039 (1.017 to 1.062) | 0.001 |
| Age | 1.325 (1.138 to 1.543) | < 0.001 |
| Overall survival time | ||
| Mitotic index | 1.045 (1.022 to 1.069) | < 0.001 |
| Age | 1.388 (1.167 to 1.651) | < 0.001 |
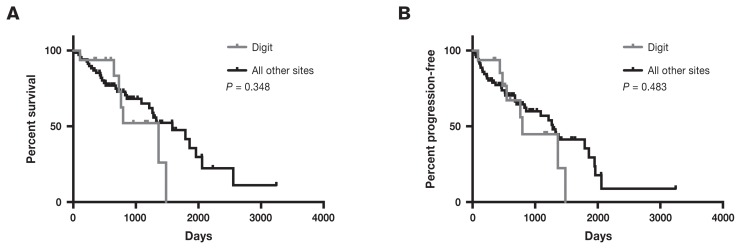
As there is some debate regarding the behavior of melanomas of the haired skin of the digits, we compared the survival time of CMM of the haired skin over the digit with all other CMM sites. Cutaneous malignant melanoma of the haired skin of the digit was defined as masses confined to the haired skin overlying the digit distal to the metacarpals or metatarsals. In total, there were 16 CMM of the haired skin of the digits. The OST for CMM of the digit was 1363 d (range: 107 to 1484 d) compared with 1584 d (range: 27 to 3246 d) for all other sites (Figure 2). The PFS was 797 d (range: 87 to 1484 d) for CMM of the digit compared with 1282 d (range: 27 to 3246 d) for CMM of all other sites. The differences in OST and PFS between CMM of the digit and all other sites were not statistically significant with P-values of 0.348 for OST and 0.483 for PFS.
Figure 2.
Kaplan-Meier curves for overall survival (A) and progression-free survival (B) divided by masses occurring on the digit compared to all other sites for dogs treated with surgery for cutaneous malignant melanoma of the haired skin.
Discussion
This study demonstrates lengthy survival times for dogs with cutaneous melanoma of the haired skin. It appears that local resection alone is adequate treatment for most CMM cases as the local recurrence rate was low (8%), as was the metastatic rate (21.8%). This information is important for clinicians and justifies the case for less aggressive adjuvant therapy in most cases, especially because the efficacy of adjuvant therapy in delaying or preventing malignant melanoma metastasis is an area of active debate (4,11,12). One prognostic factor identified in this study was MI. An MI > 20 per 10 hpf was predictive for OST and an MI > 17 was predictive of PFS; however, patients with these negative predictors still had a median OST of 1089 d and a PFS of 679 d. This MI is much higher than the previously accepted cut-off of 3, as dogs with a tumor MI ≥ 3 have decreased survival (7). Another more recent report also did not find the MI cut-off of 3 to be prognostic (13). Age above the median age of the study (9.4 y old) was also found to be significantly and independently associated with OST and PFS. This is not surprising since age was identified as an independent prognostic indicator for decreased median survival time in a recent retrospective review of 151 cases of canine oral melanoma (3). Increasing age has also been identified as an independent predictor of poor outcome in human melanoma patients (14,15). Weakened immune responses and more frequent co-morbidities are possible explanations for the more guarded prognosis in older dogs.
Interestingly, 5 of the 24 patients with documented disease progression were deemed progressive due to development of CMMs at distant cutaneous sites. This suggests that there may exist a population of patients with increased risk of developing multiple CMMs for which an underlying risk factor might be identified.
With regard to the role of adjuvant therapy, there is conflicting evidence that adjuvant carboplatin or vaccine therapy improves the outcome in canine CMM. This study suggests that adjuvant treatment with either carboplatin or vaccine-based therapy does not lead to a statistically significant increase in either OST or PFS.
In previous studies of CMM of the haired skin of the digit, there has been inconsistent evaluation, mainly inclusion with nailbed, paw pad, and in 1 study inclusion with all digit and lip melanomas, making it difficult to interpret the behavior of the masses that are purely cutaneous and affect only the haired skin overlying the digit (6,13,16). To our knowledge, there are no reports in the literature in which CMMs of the digit are clearly delineated from the nailbed and paw pad melanomas with regard to outcome. Lacking this information, CMMs of the haired skin of the digit may be treated more aggressively than necessary. In this study, we separated out the CMMs of the digit (those that were clearly defined in the medical record as affecting the haired skin overlying or distal to the metacarpals or metatarsals). In doing this, we found that these masses did not appear to behave more aggressively than CMM of other sites. A median OST of over 3 y was achieved in CMM of the digit. Although comparison among studies, especially retrospective studies, is not possible, the OST reported here is longer than other previously reported survival times for all digit melanomas, which is approximately 365 d (16,17). It should be noted that the number of patients with CMM of the haired skin of the digit in this study was relatively low (n = 16).
The retrospective nature of this study limits its broad application. In particular, the lack of centralized pathology review and the inability to evaluate all tumor specimens for other histopathologic factors such as margins and other features that have been previously linked to prognosis such as nuclear atypia, Ki-67 and degree of pigmentation, is a weakness (2). To evaluate the effect of close margins on outcome, we included any case in which the pathology report clearly stated that the mass was incompletely excised and any case with a lateral margin < 5 mm. The use of a 5-mm lateral margin was arbitrary and may represent a source of bias. Similarly, inconsistent follow-up and lack of definitive information with regard to tumor metastasis and recurrence at the time of death (i.e., via necropsy) limits any definitive conclusions; however, even if patients died with undetected metastasis or local recurrence, the OST was long.
In conclusion, the behavior of CMM of the haired skin in dogs appears to take a predominantly benign course, and local control with surgery can lead to long survival times. In cases with an MI > 20, more aggressive management may be considered, although the effectiveness of this is unknown as treatment with adjuvant therapy (vaccine or carboplatin) did not enhance survival times.
Figure 1.
Kaplan-Meier curves for overall survival (A) and progression-free survival (B) according to mitotic index for dogs treated with surgery for cutaneous malignant melanoma of the haired skin.
Acknowledgment
The authors thank Sarah Wetzel for her help in abstracting case information. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bergman P, Kent M, Farese J. Melanoma. In: Withrow SJ, Vail DM, Page RL, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 5th ed. St. Louis, Missouri: Elsevier; 2013. pp. 321–334. [Google Scholar]
- 2.Smedley RC, Spangler WL, Esplin DG, et al. Prognostic markers for canine melanocytic neoplasms: A comparative review of the literature and goals for future investigation. Vet Pathol. 2011;48:54–72. doi: 10.1177/0300985810390717. [DOI] [PubMed] [Google Scholar]
- 3.Boston SE, Lu X, Culp WT, et al. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001–2012) J Am Vet Med Assoc. 2014;245:401–407. doi: 10.2460/javma.245.4.401. [DOI] [PubMed] [Google Scholar]
- 4.Grosenbaugh DA, Leard AT, Bergman PJ, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–1638. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- 5.Tuohy JL, Selmic LE, Worley DR, Ehrhart NP, Withrow SJ. Outcome following curative-intent surgery for oral melanoma in dogs: 70 cases (1998–2011) J Am Vet Med Assoc. 2014;245:1266–1273. doi: 10.2460/javma.245.11.1266. [DOI] [PubMed] [Google Scholar]
- 6.Spangler WL, Kass PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. 2006;43:136–149. doi: 10.1354/vp.43-2-136. [DOI] [PubMed] [Google Scholar]
- 7.Bostock DE. Prognosis after surgical excision of canine melanomas. Vet Pathol. 1979;16:32–40. doi: 10.1177/030098587901600103. [DOI] [PubMed] [Google Scholar]
- 8.Laprie C, Abadie J, Amardeilh MF, Net JL, Lagadic M, Delverdier M. MIB-1 immunoreactivity correlates with biologic behaviour in canine cutaneous melanoma. Vet Dermatol. 2001;12:139–147. doi: 10.1046/j.1365-3164.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 9.Bongiovanni L, D’Andrea A, Porcellato I, et al. Canine cutaneous melanocytic tumours: Significance of beta-catenin and survivin immunohistochemical expression. Vet Dermatol. 2015;26:270–e259. doi: 10.1111/vde.12211. [DOI] [PubMed] [Google Scholar]
- 10.Weiss SA, Hanniford D, Hernando E, Osman I. Revisiting determinants of prognosis in cutaneous melanoma. Cancer. 2015;121:4108–4123. doi: 10.1002/cncr.29634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockley LK, Cooper MA, Bennett PF. Malignant melanoma in 63 dogs (2001–2011): The effect of carboplatin chemotherapy on survival. N Z Vet J. 2013;61:25–31. doi: 10.1080/00480169.2012.699433. [DOI] [PubMed] [Google Scholar]
- 12.Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M, Obradovich JE. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet Comp Oncol. 2013;11:219–229. doi: 10.1111/vco.12057. [DOI] [PubMed] [Google Scholar]
- 13.Schultheiss PC. Histologic features and clinical outcomes of melanomas of lip, haired skin, and nail bed locations of dogs. J Vet Diagn Invest. 2006;18:422–425. doi: 10.1177/104063870601800422. [DOI] [PubMed] [Google Scholar]
- 14.Chao C, Martin RC, Ross MI, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004;11:259–264. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald JB, Dueck AC, Gray RJ, et al. Malignant melanoma in the elderly: Different regional disease and poorer prognosis. J Cancer. 2011;2:538–543. doi: 10.7150/jca.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marino DJ, Matthiesen DT, Stefanacci JD, Moroff SD. Evaluation of dogs with digit masses: 117 cases (1981–1991) J Am Vet Med Assoc. 1995;207:726–728. [PubMed] [Google Scholar]
- 17.Wobeser BK, Kidney BA, Powers BE, et al. Diagnoses and clinical outcomes associated with surgically amputated canine digits submitted to multiple veterinary diagnostic laboratories. Vet Pathol. 2007;44:355–361. doi: 10.1354/vp.44-3-355. [DOI] [PubMed] [Google Scholar]