Figure 1.
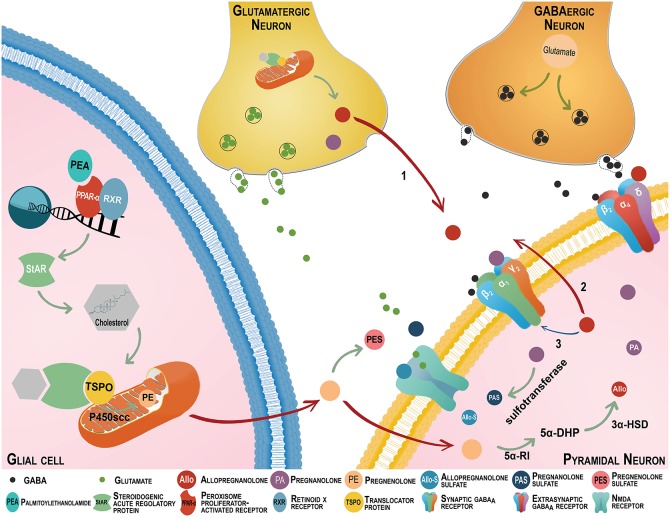
The endocannabinoid and neurosteroid systems cross-talk regulates emotional behavior. The neurosteroids, allopregnanolone (Allo) and its equipotent isomer pregnanolone (PA) are primarily synthesized in glutamatergic corticolimbic neurons and upon secretion; they may act at GABAA receptors located on cell bodies or dendrites of distal pyramidal neurons (Arrow 1). They may also act at GABAA receptors located on glutamatergic neurons' dendrites or cell bodies by an autocrine mechanism (Arrow 2), or may access and act at the intracellular sites of GABAA receptors located in glutamatergic neurons that produced allopregnanolone itself (Arrow 3) (Agís-Balboa et al., 2006, 2007; Pinna et al., 2008). Allopregnanolone plays a central neuromodulatory role in facilitating the action of GABA at GABAA receptors (a primary target of anxiolytics) and in the fine-tuning of the receptor for agonists and GABAmimetic agents (Pinna et al., 2000). The finding that allopregnanolone facilitates the efficacy of GABAA receptor allosteric modulators substantiates its endogenous physiological relevance (Pinna et al., 2000, 2008; Guidotti et al., 2001). Importantly, GABAA receptors composed by α,β,γ subunits are the most common configuration in the synaptic membranes and they are responsible for the inhibitory phasic currents. These receptors are benzodiazepine-sensitive but show lower sensitivity to GABA and allopregnanolone (Nusser and Mody, 2002). The GABAA receptors including α,β,δ subtypes are mostly extrasynaptic and mediate inhibitory tonic currents. Of note, they are not sensitive to benzodiazepines and show low efficacy for GABA, however, allopregnanolone increase their efficacy (Stell et al., 2003; Shu et al., 2012). The efficacy of GABAergic neurosteroids is greatly enhanced for this receptor combination (Brown et al., 2002; Nusser and Mody, 2002; Wohlfarth et al., 2002). Remarkably, protracted stress favors a GABAA receptor composition with high sensitivity for allopregnanolone and its analogs (Locci and Pinna, 2017a). Following the action of sulphotransferase, allopregnanolone, and pregnanolone can be transformed into allopregnanolone sulfate (Allo-S) and pregnanolone sulfate (PAS). These sulfated steroids can be measured by gas chromatography-mass spectrometry in serum, CSF, and brain of patients or rodents in concentrations consistent with a physiological role in modulating neurotransmitter systems (Smith et al., 2014; Locci and Pinna, 2017b). Recently, pregnanolone sulfate has been shown to inhibit NMDA receptors. Pregnanolone sulfate can accumulate in plasma membranes and may accesses binding sites that are located at NMDA receptors (Borovska et al., 2012). Importantly, pregnanolone sulfate, and probably allopregnanolone sulfate, is highly potent at inhibiting tonic rather than synaptically mediated NMDA receptor neurotransmissions. While synaptic NMDA receptors play a pivotal role in synaptic plasticity, learning and memory, as well as in synaptogenesis, tonic-mediated NMDA receptor neurotransmission is mostly involved with excitotoxicity. Thus, the effects of pregnanolone sulfate negative modulation of tonic-mediated NMDA receptor neurotransmission have relevance for neuroprotection (Vyklicky et al., 2016). By this mechanism, these allopregnanolone and pregnanolone sulfated derivatives may play a role in the regulation of cognitive processes and of emotional behavior (reviewed in Locci and Pinna, 2017a). There is growing evidence that the intracellular peroxisome proliferator-activated receptor (PPAR-α) is also a cannabinoid target (depicted on the bottom right). PPAR-α heterodimerize with the retinoid X receptor (RXR) and binds to the consensus regions on the target gene promoters and initiates transcription (Neumeister, 2013). Given that endoannabinoids activate PPAR-α (Marsicano et al., 2002; Pistis and Melis, 2010), the activation of these nuclear receptors represents a novel mechanism by which cannabinoids may modulate behavior. The endocannabinoid congener, N-palmitoylethanolamine (PEA) is a PPAR-α endogenous agonist, which is decreased in PTSD patients (Wilker et al., 2016). Recent preclinical findings showed that supplementing PEA in rodent PTSD models improves emotional behavior by enhancing allopregnanolone biosynthesis in corticolimbic glutamatergic neurons. This effect is mimicked by PPAR-α agonists and prevented by allopregnanolone biosynthetic enzyme blockers and by deletion of the PPAR-α gene (Locci and Pinna, 2017b). Thus, anxiolytic, anti-aggressive and anti-fear effects of PEA and synthetic PPAR-α agonists may relate to an induction of corticolimbic allopregnanolone's biosynthetic enzymes. This may result in potentiation of GABAA receptor and, possibly, in an inhibition of tonic-mediated NMDA signal transduction associated with improved behavioral dysfunction. Stress effects on PEA levels and probably expression of PPAR-α may result in the downregulation of allopregnanolone's biosynthetic enzyme expression and allopregnanolone levels. The interface of the endocannabinoid and neurosteroid systems may provide an important biomarker axis to selectively predict, diagnose, and establish the best individualized treatment selection for PTSD patients.