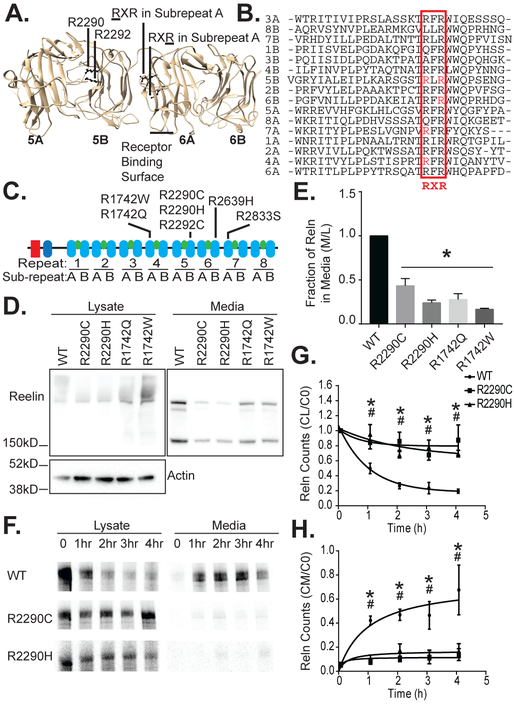
Figure 1. The RELN R2290C and other ASD mutations reside in a conserved RXR consensus and impair Reelin secretion.
(A) Rendering of Reelin repeat 5 and 6 crystal structure (PDB 2E26; Chimera). The positions of the R2290C and R2292C mutations in the RXR motif of 5B are shown facing into the hydrophilic pore of RELN repeat 5. The corresponding locations of the RXR motif in an A repeat are also indicated. The position of the receptor-binding loops in 6A are indicated. (B) Alignment of the conserved region of the Reelin sub-repeat domains (Clustal Omega). The RXR consensus element is boxed and indicated below. (C) Schematic of Reelin structure showing an F-spondin homology domain, an N-terminal domain unique to Reelin, and 8 Reelin repeat domains. Each Reelin repeat domain contains an A and B sub-repeat domain (blue ovals) connected by an EGF domain (green ovals). ASD-associated mutations are indicated above their respective repeat. (D) Less Reelin was detected in media of HeLa cells transfected with full-length mutant (R2290C, R2290H, R1742Q, or R1742W) as compared to wild-type (WT) Reelin by Western blotting (anti-Reelin G10), whereas equal or greater amounts were observed in the cell lysates. One full-length (>400 kD), smeared Reelin band was observed in the cell lysates and three products (full length and two cleavage products: 410, 380, and 180 kD) were observed in the media, as expected (Jossin et al. 2004). Reelin is not expressed in vector-alone transfected HeLa cells (not shown). (E) The ratio of Reelin signal in the media (M) to Reelin lysate signal (L) was significantly greater for WT than mutant Reelin (n=4, error bars ± SEM, and * p value < 0.05 by 1-way ANOVA with Dunnett’s post hoc test). (F) WT Reelin metabolically labeled with 35S-methionine was most abundant in 293 cell lysates at the beginning of a cold chase, but by two hours the majority was detected in the cell media. In contrast, the majority of radiolabeled R2290C and R2290H mutant RELN remained in cell lysates over a 4h time course, as detected by phosphor-imaging of immunoprecipitated Reelin (anti-G10). (G) In the 293 cell lysate the Reelin counts (CL) at times indicated relative to counts at time 0 (C0) decreased more rapidly for WT Reelin than the R2290C and R2290H mutants (n=3, error bars ± SEM, * p<0.05 R2290C, # p<0.05 R2290H by 2-way ANOVA with Dunnett’s post hoc test). (H) In the media the Reelin counts (CM) at indicated times relative to lysate counts at time 0 (C0) increased more rapidly for WT than R2290C or R2290H (n=3, error bars ± SEM, * p<0.05 R2290C, # p<0.05 R2290H by 2-way ANOVA with Dunnett’s post hoc test).