Figure 2. The LINC00116-Derived Microprotein Mitoregulin Localizes to Inner Mitochondrial Membranes and Binds Cardiolipin.
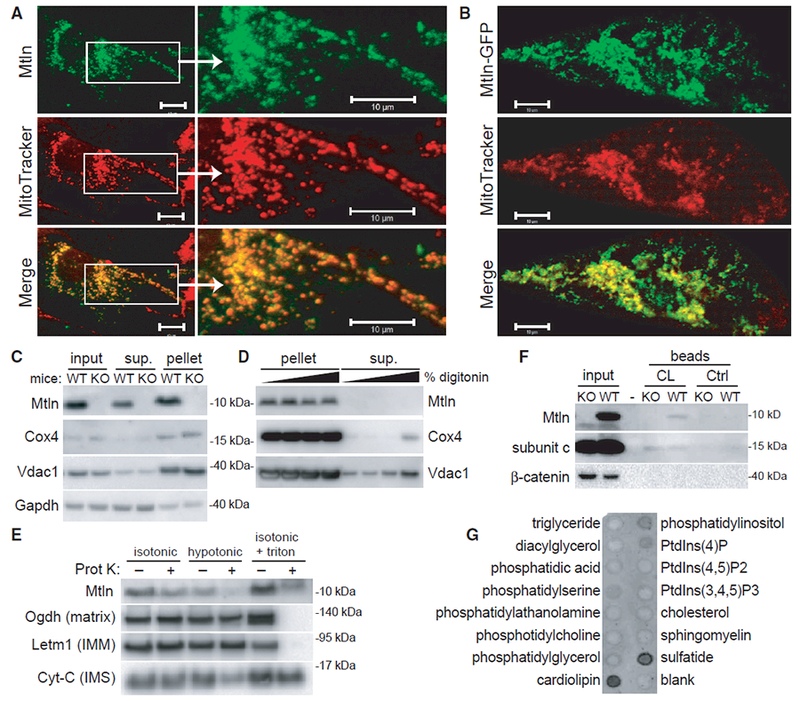
(A and B) Wild-type (A) and GFP-tagged (B) human Mtln were expressed in neonatal rat cardiomyocytes, and co-localization with MitoTracker red was evaluated. Representative photomicrographs are shown. Scale bars, 10 μm.
(C) Mitochondrial pellets were isolated from wild-type (WT) or Mtln-knockout (KO) C2C12 myoblast cells, and western blot was performed on various fractions.
(D) Mitochondrial pellets harvested from WT or Mtln-KO skeletal muscle tissues were treated with increasing digitonin concentrations to release OMMs, and pellet and supernatant fraction fractions were subjected to western blot analysis. Cox4 and Vdac1 are known IMM and OMM proteins, respectively. Gapdh is a cytosolic protein known to associate with mitochondria in some cases.
(E) Mitochondrial pellets harvested from WT skeletal muscle tissues were resuspended in isotonic, hypotonic, or isotonic plus triton buffers in the absence or presence of proteinase K and subjected to western blot analysis. Proteins with known localization to various mitochondrial compartments (e.g., matrix, IMM, and intermembrane space [IMS]) were evaluated as controls.
(F) Western blot analysis performed on WT and Mtln-KO cardiac tissue lysates subjected to pull-down assay using cardiolipin (CL)-coated or control beads. Subunit c, a known cardiolipin-binding protein, serves as the positive control.
(G) Lipid-strip binding assay performed using synthetic Mtln protein followed by anti-Mtln immunoblot.
