Fig. 1.
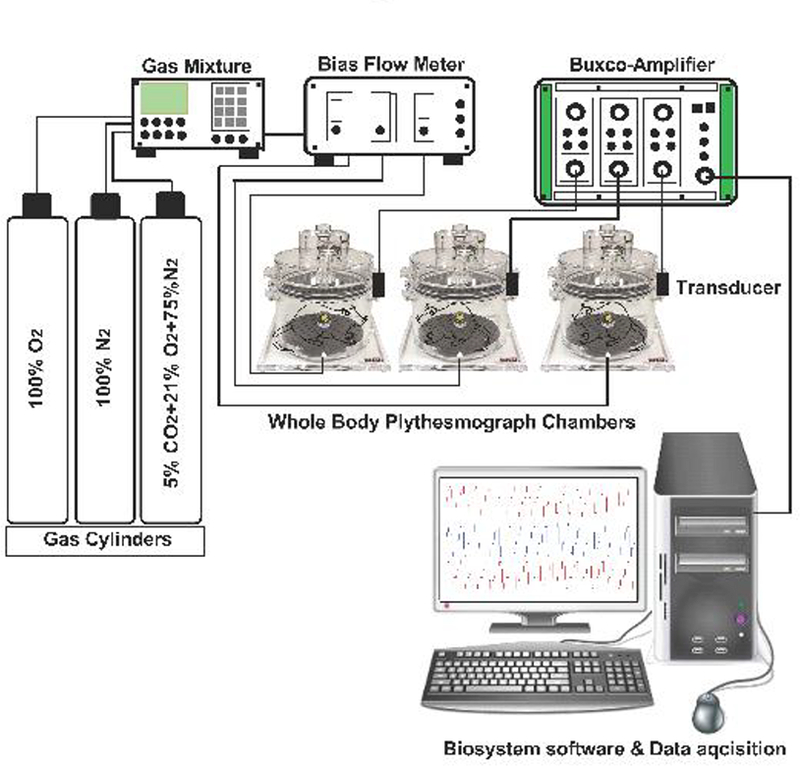
Schematic of Whole-body plethysmograph setup. Sham-operated (SHAM) and bilateral carotid sinus nerve transected (CNSX) rats were gradually acclimatized to the whole-body plethysmography chamber with a bias chamber air flow. A bias chamber air flow of at least 2 L/min was generated by connecting the chambers to a constant flow vacuum source. However, during normoxia, hypoxia and hypercapnia protocols, a bias flow of room air (PO2 of 21%), hypoxic (PO2 of 10%) and hypercapnic (PCO2 of 5%) gases were generated using a gas mixture. Gas mixture receives inflow from 100% N2, 100% O2 and 10% CO2 cylinders. Gas flow to each plethysmograph chamber (2 L/min) was controlled using flow meters. A respiratory waveform was generated from the expansion and contraction of the air that was exchanged between the rat and the chamber. The cyclic change in air volume during the respiratory cycle elicited oscillating airflow across a calibrated pneumotach in the wall of the plethysmograph chamber. Each pneumotach was calibrated (5.0 mL volume delivered in triplicate) on each study day prior to placing the rats in the chambers. At least 1 hour was permitted for rats to acclimate to the chamber before data collection began. Respiratory waveforms were amplified with Buxco-Amplifiers and captured continuously and stored using FinePointe software later data analysis. Barometric pressure, chamber temperature, chamber partial pressure of water, and body temperature were used to calculate a corrected tidal volume. The first three variables were assumed to be constant throughout the study at 740 mmHg, 25 °C, and 23.7 mmHg, respectively. Minute ventilation was calculated as the product of tidal volume and respiratory frequency.
