Figure 5. Hsp20 Regulates PPARγ Protein Ubiquitination and Stability in Isolated Adipocytes.
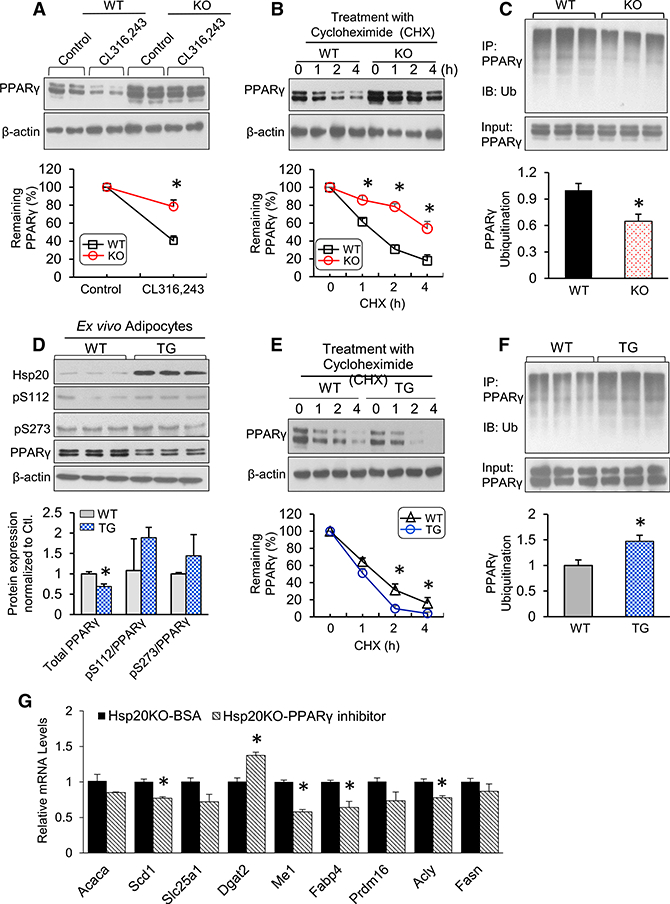
(A) Protein levels of PPARγ in differentiated WT or KO adipocytes upon CL316,243 treatment. The expression level of PPARγ in control cells was set to 100%.
(B) The differentiated cells were treated with cycloheximide (CHX) (5 μM) for 0, 1, 2, or 4 hr. Immunoblot analysis results of PPARγ are reported as a percentage of the value at 0 hr.
(C) The differentiated cells were treated with MG132 (10 μM) for 4 hr, and protein extracts were immunoprecipitated with anti-PPARγ, followed by immunoblotting with anti-ubiquitin (Ub).
(D) Immunoblot analysis and quantification of total PPARγ and phospho-PPARγ at Ser-112 and Ser-273 on day 8 of SVC differentiation.
(E) The differentiated cells were treated with CHX (5 μM) for 0, 1, 2, or 4 hr. Immunoblot analysis of PPARγ was conducted, and quantification results are indicated as a percentage of the value at 0 hr.
(F) The differentiated cells were treated with MG132 (10 μM) for 4 hr, and protein extracts were immunoprecipitated with anti-PPARγ, followed by immunoblotting with anti-Ub.
(G) mRNA expression of lipogenic genes in Hsp20-KO adipocytes treated with PPARγ (5 μM, 8 hr).
*p < 0.05. Data are represented as the mean ± SEM. See also Figures S5 and S6.
