Abstract
Background
Sexual dimorphism in cardiac sympathetic outflow has recently gained attention in the context of Takotsubo cardiomyopathy. Previous studies suggest that there are sex- and age-dependent differences in peripheral autonomic control, however, data on cardiac-specific sympathetic activation in aged women and men are lacking.
Methods and results
Regional quantitative analysis of cardiac fluorine-18 (18F)- Dihydroxyphenylalanine (DOPA) uptake was retrospectively performed in 133 patients (69 females, mean age 52.4±17.7 years) referred for assessment of neuroendocrine tumours (NET) by Positron-Emission-Tomography. Cardiac 18F-DOPA uptake was significantly higher in women as compared to men (1.33±0.21 vs. 1.18±0.24, p<0.001). This sex-difference was most pronounced in the apical region of the left ventricle (LV, 1.30±0.24 in women vs. 1.13±0.25 in men, p<0.001) and in individuals >55 years of age (1.39±0.25 in women vs. 1.09±0.24 in men, p<0.001). Women showed a prominent increase in myocardial 18F-DOPA uptake with age with the strongest increase seen in the LV apical region (r = 0.34, p = 0.004). Accordingly, sex and age were selected as significant predictors of LV apical 18F-DOPA uptake in a stepwise linear regression model. No age-dependent changes of cardiac 18F-DOPA uptake were observed in men or in the right ventricular region.
Conclusion
Our study suggests that aging is related to sex-specific changes in regional cardiac sympathetic activity. Future studies will have to assess whether the increase in LV apical 18F-DOPA uptake with age in women is of pathogenic relevance for the higher susceptibility of postmenopausal women to conditions associated with increased sympathetic activity.
Introduction
Cardiovascular disease is the leading cause of death and disease burden in both, women and men, in the western world. While cardiovascular mortality rates have rapidly declined since the late 1970s in men, death rates in women have not improved to the same extend. In fact, the disease is becoming more common in women, in particular in young women, and cardiovascular deaths in women currently exceed those in men.[1, 2] However, there is currently only limited data on sex-specific pathogenesis, management, and outcomes of cardiovascular disease.
Sexual dimorphism in cardiac sympathetic outflow has recently gained increasing attention in the context of Takotsubo cardiomyopathy or cardiac syndrome X.[3–6] The worse outcomes observed in women with cardiovascular disease as well as their higher susceptibility to cardiac injury during high-stress situations implies that sex-differences in autonomous nervous control of the cardiovascular system might be pathogenetic.[7, 8] However, there is a lack of data on sex- and age-specific cardiac sympathetic activity in normal individuals and very little information about regional norepinephrine turnover, uptake, and metabolism in the human myocardium is available in the literature. Indeed, previous quantification of sympathetic activity in humans was mainly obtained by unspecific approaches such as power spectral analysis of heart rate variability (HRV), measurement of muscle sympathetic nervous activity (MSNA) or quantification of circulating catecholamine levels and have produced widely varying results.[9, 10] Notably, while MSNA seems to be a good indicator of cardiac or renal sympathetic activity and vasoconstrictor tone in men, studies investigating this association in women are sparse and have failed to detect a significant correlation between MSNA and cardiac output or vasoconstrictor tone.[11]
Measurement of regional cardiac sympathetic activity by sympathetic neurotransmitter radionuclide analogues (e.g. 123I-Metaiodobenzylguanidine [MIBG]) has been shown to add incremental prognostic value in predicting disease progression in heart failure patients beyond that provided by traditional markers.[12] Hence, regional dysfunction of the sympathetic nervous system might add important prognostic information about cardiac vulnerability of women and men and may help to direct preventive strategies and therapy. Besides 123I-MIBG for scintigraphy, an 18F labelled Positron-Emission-Tomography (PET) tracer was developed using the catecholamine precursor fluorine-18 (18F)-Dihydroxyphenylalanine (18F-DOPA) to image active paragangliomas, tumours derived from the autonomous nervous system.[13]
Given the different susceptibility of postmenopausal women and older men to cardiac conditions associated with sympathetic dysregulation, we hypothesized that sex- and age-dependant differences exist with regard to cardiac autonomic control. As the superior spatial resolution of PET allows to assess even small differences in tracer uptake, the primary aim of our study was the quantification of regional cardiac 18F-DOPA uptake in men and women free of cardiovascular disease at different ages.
Methods
Study population
Cardiac uptake of 18F-DOPA was retrospectively assessed in 133 individual patients (69 females, mean age 52.4±17.7 years, range 1–84 years) who underwent functional imaging with 18F-DOPA PET-CT for evaluation of a known or suspected NET at our institution between February 2007 and March 2016. Repeated 18F-DOPA exams were excluded from our study. Information on medical history and cardiovascular risk factors (hypertension, hyperlipidemia, diabetes, smoking, family history of premature CAD) were obtained from electronic patient records. Subjects with structural heart disease including heart failure and valvular heart disease, known obstructive coronary artery disease, previous myocardial infarction, previous coronary artery bypass grafting or percutaneous coronary intervention, hypertension or diabetes mellitus were excluded from our analysis. The study conforms to the principles outlined in the 1964 Declaration of Helsinki and was approved by the local cantonal ethics committee in Zurich, Switzerland (BASEC No. 2017–01112). The need to obtain informed consent was waived by the ethics committee due to the retrospective nature of the study.
Image acquisition and reconstruction
DOPA is a neutral amino acid that resembles natural L-DOPA (dopamine precursor). It enters the catecholamine metabolic pathway of endogenous L-DOPA in the brain and peripheral tissues and can be labeled with 18F (half-life 110 min) for PET imaging. Incerased uptake of 18F-DOPA is seen in tissues with high activities of L-DOPA decarboxylase. 18F-DOPA-PET was used at our institution after its formal approval for use in Europe (November 2006). Patients were asked to fast for at least 4–6 h before 18F-DOPA injection. 18F-DOPA-PET was performed 45 min after injection 18F-DOPA (mean 202.8±36.7 MBq, range 25–263 MBq) into a peripheral vein.[14] Images were acquired in 3D mode on different scanners (Discovery VCT or Discovery RX (GE-Healthcare, Milwaukee, WI, USA). The imaging protocol consisted of a scout view followed by low-dose CT acquisition for attenuation correction and subsequent PET acquisition. PET emission scans were acquired from the base of the skull to mid-thigh during 20 min (5–7 bed positions of 2–3 min each). Iterative reconstruction and CT-based attenuation correction were used. PET and CT images were fused using a dedicated software package (AW 5.0 GE-Healthcare). PET scans were acquired without premedication with carbidopa.
Data analysis
Reconstructed data were transferred to an external workstation (Advantage AW 4.4, GE Healthcare) for analysis. On reformatted horizontal long-axis slices, the left ventricle (LV) and right ventricle (RV) were subdivided into a total of six segments (LV basal and midventricular lateral wall, LV basal and midventricular septum, LV septal and lateral apical segments, and RV basal and midventricular lateral wall). Cardiac segments in our study were defined based on the recommendations of the American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging.[15] A semiquantitative analysis of regional 18F-DOPA uptake was performed by placing a circular volume of interest (VOI) of 2.3cm3, automatically generated by the computer, into these four segments. The size of the VOI was standardized for all images by using a semi-automatic volume analysis tool (GE Healthcare, Milwaukee, USA). A semiquantitative uptake analysis using the upper limit of the standardized uptake value (SUVmax) and the averaged standardized uptake value (SUVmean) was used to quantify 18F-DOPA uptake in these regions. SUVmax and SUVmean were normalized to blood pool 18F-DOPA activity measured in the aortic arch of each patient (= SUVmax-N and SUVmean-N).
Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. Data were stratified for sex and age. The age of 55 years was used as a cut-off value to differentiate between younger and older individuals. This cut-off value was chosen given that most women undergo menopause between 45 and 55 years and that, at the age of 55 years, 95% of women are postmenopausal.[16] Prior to analyses, basic assumptions were checked. Student’s t-test, Mann-Whitney test, analysis of variance (ANOVA) or Kruskal-Wallis test were used for group comparisons of continuous variables. For comparison of different age- and sex-groups, p-values were adjusted by the Bonferroni correction for multiple tests. For categorical variables, chi-square tests or Fisher's exact test were used, as appropriate. Multivariate linear regression analysis was applied to assess the association of age and sex with regional cardiac sympathetic activity. All tests were two-sided, and p values below 0.05 were considered significant. Statistical analyses were performed with IBM SPSS statistics v24.0 and GraphPad Prism (v4.0, GraphPad Software, San Diego, CA).
Results
Patients characteristics
Regional quantitative analysis of cardiac 18F-DOPA uptake was performed in reconstructed PET/CT images of all 133 patients. All patients were specifically referred for 18F-DOPA PET-computed tomography (CT) for evaluation of a known or suspected pheochromocytoma (n = 26), carcinoid (n = 15), thyroid carcinoma (n = 18), or extra-adrenal paraganglioma (n = 60, Table 1) according to clinical, biochemical or radiological data. In two cases the reason for 18F-DOPA PET imaging was the presence of a carcinoma of unknown primary origin, two patients were referred for evaluation of ectopic adrenocorticotropic hormone production and 10 cases had suspicious hepatic, pancreatic and adrenal masses (Table 1). In 67 (50.4%) patients 18F-DOPA scans were positive for paraganglioma. When data were stratified by sex, no significant differences with regard to baseline characteristics were observed between men and women (p = NS, Table 1), except for BMI, which was significantly higher in men (p<0.001, Table 1). Clinical indications for 18F-DOPA PET referral and patients characteristics stratified by sex are depicted in Table 1.
Table 1. Patient baseline, 18F-DOPA PET acquisition characteristics, regional cardiac 18F-DOPA uptake, and clinical indications for 18F-DOPA PET imaging.
| Baseline characteristics | Total n = 133 | Womenn = 69 | Menn = 64 | p-value |
|---|---|---|---|---|
| Age (years), mean±SD | 52.4±17.7 | 52.9±19.2 | 52.0±16.1 | 0.8 |
| BMI, mean±SD | 22.7±6.8 | 20.4±5.4 | 25.2±7.2 | <0.001 |
| 18F-DOPA positive scan, n(%) | 67(50.4) | 31(44.9) | 36(56.3) | 0.2 |
| Injected 18F-DOPA dose (MBq), mean±SD | 202.8±36.7 | 204.4±40.1 | 201.2±32.9 | 0.6 |
| Reason for referral, n(%) | 0.3 | |||
| Clinical suspicion of carcinoid | 5(3.8) | 2(2.9) | 3(4.7) | |
| Clinical suspicion of pheochromocytoma | 13(9.8) | 8(11.6) | 5(7.9) | |
| Clinical suspicion of extra-adrenal paraganglioma | 13(9.8) | 6(8.7) | 7(11.1) | |
| Treatment control carcinoid | 10(7.5) | 7(10.1) | 3(4.7) | |
| Treatment control thyroid carcinoma | 18(13.5) | 9(13.0) | 9(14.3) | |
| Treatment control pheochromocytoma | 13(9.8) | 5(7.2) | 8(12.7) | |
| Treatment control extra-adrenal paraganglioma | 47(35.3) | 26(37.7) | 21(33.3) | |
| Carcinoma of unknown primary origin | 2(1.5) | 1(1.4) | 1(1.5) | |
| Ectopic ACTH production | 2(1.5) | 0(0) | 2(3.2) | |
| Suspicious mass | 10(7.5) | 6(8.7) | 4(6.3) | |
| Medical treatment | ||||
| Antiadrenergic compounds | 26(19.5) | 17(24.6) | 9(14.1) | 0.12 |
| Immunosuppressive/antiinflammatory agents | 13(9.8) | 7(10.1) | 6(9.4) | 0.88 |
| Somatostatin analogues | 16(12) | 8(11.6) | 8(12.5) | 0.87 |
| Thyroid hormone receptor agonists | 18(13.5) | 10(14.5) | 8(12.5) | 0.74 |
| Total myocardial 18F-DOPA uptake (SUVmax-N), mean±SD | 1.26±0.24 | 1.33±0.21 | 1.18±0.24 | <0.001 |
| Total myocardial 18F-DOPA uptake (SUVmean-N), mean±SD | 0.86±0.17 | 0.94±0.15 | 0.78±0.14 | <0.001 |
| Total LV 18F-DOPA uptake (SUVmax-N), mean±SD | 1.31±0.26 | 1.39±0.24 | 1.23±0.26 | <0.001 |
| Total LV 18F-DOPA uptake (SUVmean-N), mean±SD | 0.90±0.18 | 0.98±0.17 | 0.80±0.15 | <0.001 |
| LV-mid-ventricular 18F-DOPA uptake (SUVmax-N), mean±SD | 1.32±0.25 | 1.39±0.24 | 1.25±0.25 | <0.001 |
| LV-mid-ventricular 18F-DOPA uptake (SUVmean-N), mean±SD | 0.91±0.17 | 0.99±0.15 | 0.83±0.14 | 0.001 |
| RV 18F-DOPA uptake (SUVmax-N), mean±SD | 1.09±0.21 | 1.14±0.20 | 1.02±0.20 | 0.001 |
| RV 18F-DOPA uptake (SUVmean-N), mean±SD | 0.77±0.16 | 0.82±0.12 | 0.71±0.17 | <0.001 |
| LV-apical 18F-DOPA uptake (SUVmax-N), mean±SD | 1.28±0.28 | 1.30±0.24 | 1.13±0.25 | <0.001 |
| LV-apical 18F-DOPA uptake (SUVmean-N), mean±SD | 0.88±0.19 | 0.94±0.18 | 0.75±0.16 | <0.001 |
NET, neuroendocrine tumor; ACTH, adrenocorticotropic hormone; SUVmax-N, upper limit of the standardized uptake value normalized to blood pool; SUVmean-N, averaged standardized uptake value normalized to blood pool; RV right ventricular; LV, left ventricular. Values are indicated as mean±standard deviation (SD) or n(%). P-values are indicated for women vs men.
Overall cardiac 18F-DOPA uptake in women and men
As previously reported, mild 18F-DOPA uptake is apparent in the myocardium, peripheral muscles, esophagus, and in some cases in the mammary glands.[14] Overall cardiac 18F-DOPA uptake was significantly higher in women as compared to men (p<0.001, Table 1) and increased significantly with age in women (Pearson r = 0.32, p = 0.008) but not in men (Pearson r = -0.003, p = 0.97, data not shown). When overall cardiac 18F-DOPA uptake was measured in both, 18F-DOPA-negative for NET (n = 66) and 18F-DOPA-positive for NET (n = 67) patients, no differences in myocardial 18F-DOPA uptake were found (p = NS, data not shown). The latter was true for both, men and women (p = NS, data not shown). Similarly, when patients were stratified by medical treatment including antiadrenergic therapy, immunosuppressive/anti-inflammatory agents, somatostatin analogues, and thyroid hormone receptor agonists, no significant differences in myocardial 18F-DOPA activity were found in patients with and without treatment (1.30±0.23 vs 1.25±0.24 SUVmax-N, p = 0.37 for antiadrenergic therapy; 1.29±0.29 vs 1.25±0.23 SUVmax-N, p = 0.46 for somatostatin analogues; 1.26±0.24 vs 1.27±0.18 SUVmax-N, p = 0.35 for immunosuppressive/anti-inflammatory agents; and 1.26±0.25 vs 1.24±0.17 SUVmax-N, p = 0.76 for thyroid hormone receptor agonists).
Sex- and age-dependent changes in regional cardiac 18F-DOPA uptake
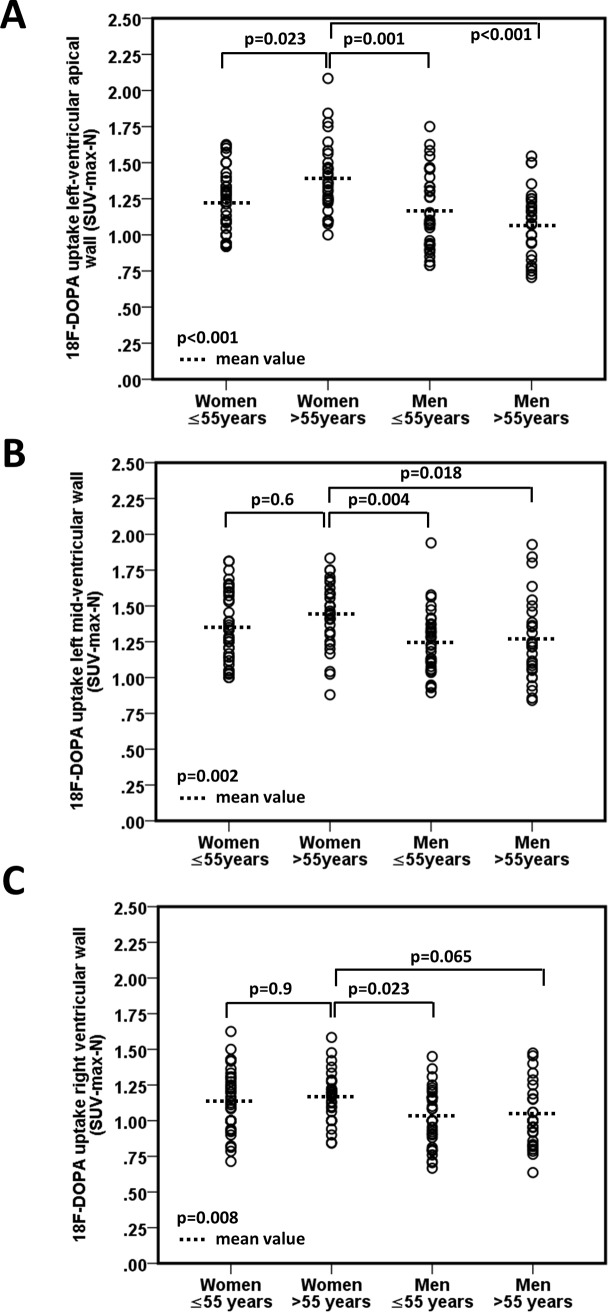
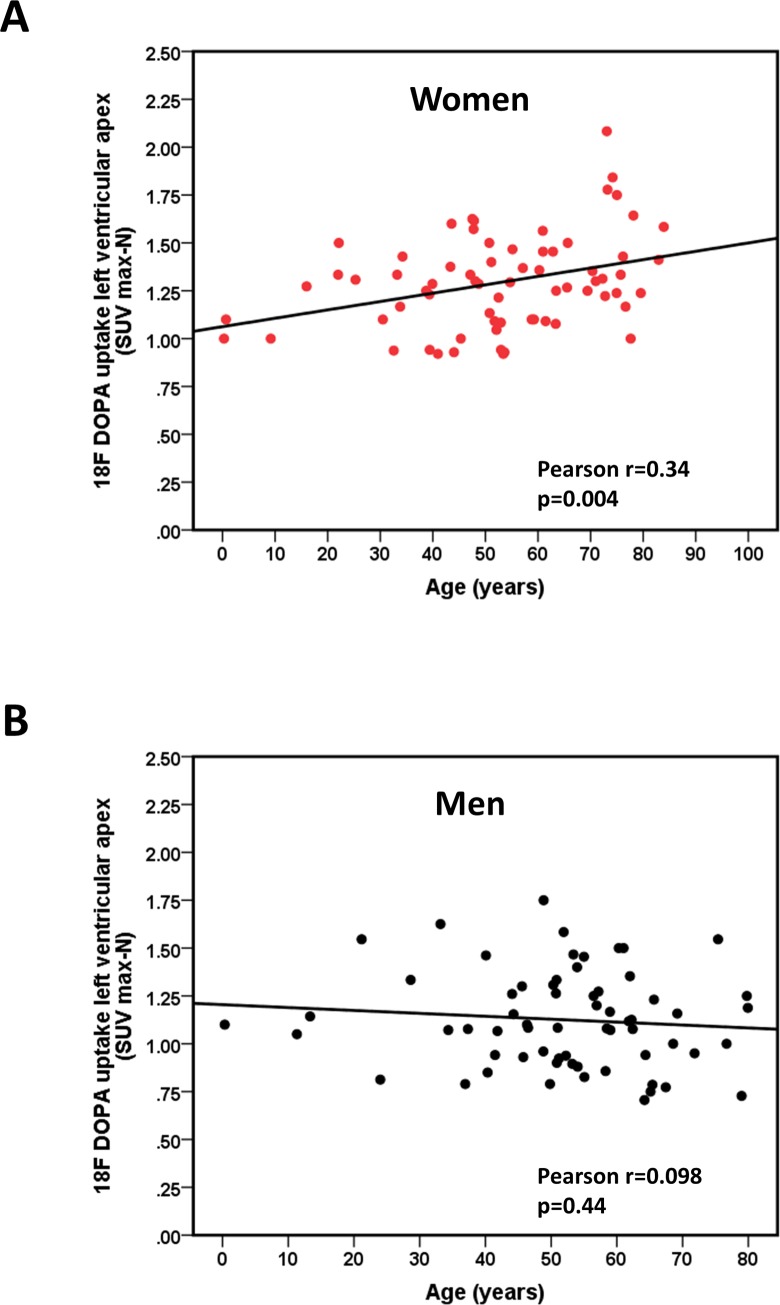
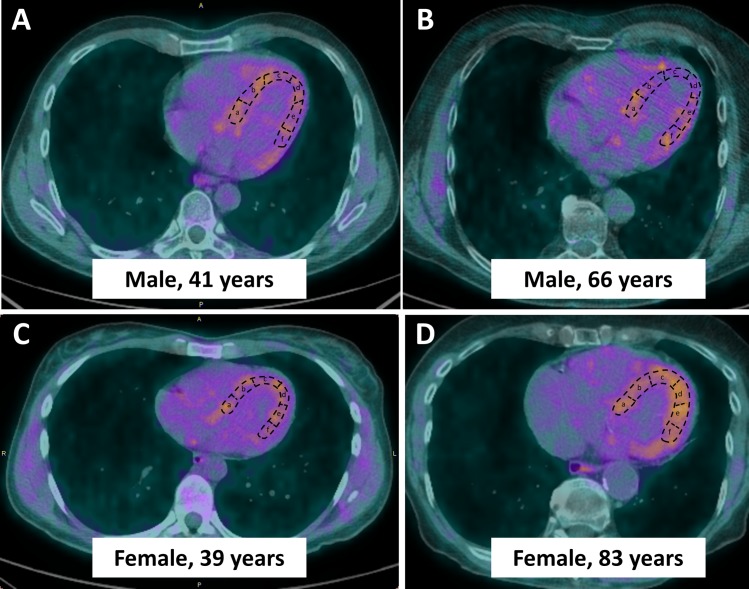
When patients were stratified by sex and age (≤ 55 and > 55 years), women >55 years had the highest cardiac 18F-DOPA uptake (p<0.05, ANOVA, Fig 1A–1C). 18F-DOPA uptake in the LV apical region increased significantly with age in women (p = 0.025 for women >55 years vs women ≤55 years, Fig 1A), while sex- and age differences in 18F-DOPA uptake were less pronounced in the left midventricular region (Fig 1B) and absent in the RV (Fig 1C). No age-dependent changes in cardiac 18F-DOPA uptake were observed in men (Fig 1A–1C). When a sub-analysis in patients >70 years was performed (n = 17 for women and n = 6 for men), we observed a further increase in LV apical 18F-DOPA uptake in women >70 years, while no such tendency was seen in older men or in other LV regions. In detail, apical 18F-DOPA uptake was 1.5±0.3 SUVmax-N in women >70 years, 1.3±0.2 SUVmax-N in women >55 and ≤70 years, and 1.2±0.2 SUVmax-N in women ≤55 years (p = 0.003). Conversely, no changes in LV apical 18F-DOPA uptake were observed in men >70 years as compared to younger age groups (1.1±0.3 SUVmax-N in men >70 years, 1.1±0.3 SUVmax-N in men >55 and ≤70 years, and 1.2±0.3 SUVmax-N in men ≤55 years (p = 0.5). Hence, a positive and significant correlation between age and LV apical 18F-DOPA uptake was seen in women (Fig 2A), while age and LV apical 18F-DOPA uptake were not associated in men (Fig 2B). In contrast, left mid-ventricular 18F-DOPA uptake was not associated with age in either sex (r = 0.2, p = 0.083 in women and r = 0.03, p = 0.8 in men, data not shown). Similar, no age-dependent changes of cardiac 18F-DOPA uptake were observed in the RV (p = NS, data not shown). Fig 3 shows representative examples of reconstructed cardiac long axis 18F-DOPA-PET-CT images of all sex and age groups.
Fig 1. Comparative analysis of myocardial 18F-DOPA uptake stratified by age (<55 years and >55 years) and sex.
18F-DOPA uptake was measured in the apical (A), left mid-ventricular (B), and right ventricular (C) region of the heart. Data are presented as dot plots. Mean values and p-values (overall and post-hoc tests) are indicated.
Fig 2. Age-dependent increase in 18F-DOPA uptake in women.
A. Correlation between age and left ventricular apical 18F-DOPA uptake (SUVmax-N) in women. Pearson correlation coefficients and p-values are indicated. B. Correlation between age and left ventricular apical 18F-DOPA uptake (SUVmax-N) in men. Pearson correlation coefficients and p-values are indicated. Pearson correlation coefficients and p-values are indicated.
Fig 3. Example of Positron-Emission-Tomography image reconstruction using the pmod cardiac PET modelling tool (pmod version 3.8, PMOD technologies LLC, Zurich, Switzerland) and quantification of 18F-DOPA uptake in the left ventricular wall.
A semiquantitative uptake analysis using the upper limit of the standardized uptake value (SUVmax-N) and the mean SUV (SUVmean-N) at the sites of physiologic uptake was performed using a planar circular region of interest of 1 cm diameter, automatically generated by the computer. Left ventricular segments in the horizontal long axis were defined as follows: a = basal septal, b = midventricular septal, c = apical septal, d = apical lateral, e = midventricular lateral, f = basal lateral. A. Cardiac long axis image of a 41 year old male patient. B. Cardiac long axis image of a 66 year old male patient. C. Cardiac long axis image of a 39 year old female patient. D. Cardiac long axis image of a 72 year old female patient. Similar thresholds were applied for all images.
Predictors of regional cardiac sympathetic activity
When sex and age were tested in a stepwise linear regression analysis with LV apical and mid-ventricular 18F-DOPA uptake being the dependent variable, and BMI and pathologic findings as predictor variables, both, sex and age were identified as significant predictors for LV apical 18F-DOPA uptake (Table 2). The probability was best explained by sex, followed by age (Table 2). When predictors for left mid-ventricular 18F-DOPA uptake were tested, sex remained a significant predictor for left mid-ventricular 18F-DOPA uptake, while age was not selected by this model (Table 3).
Table 2. Stepwise linear regression model for left-ventricular apical 18F DOPA uptake (n = 133).
| Independent variables | B coefficient (SE) | p-value |
|---|---|---|
| Male Sex | -0.15 (0.045) | 0.003 |
| Age | 0.004 (0.001) | 0.005 |
Stepwise method was performed among age, sex, body mass index (BMI) and pathologic findings on 18F-DOPA scan. Only variables staying in the final model are presented. SE, standard error.
Table 3. Stepwise linear regression model for left mid-ventricular 18F DOPA uptake (n = 133).
| Independent variables | B coefficient (SE) | p-value |
|---|---|---|
| Male Sex | -0.11 (0.047) | 0.01 |
Stepwise method was performed among age, sex, body mass index (BMI) and pathologic findings on 18F-DOPA scan. Only variables staying in the final model are presented. SE, standard error.
Discussion
In this retrospective single centre study, we performed a sex- and age-specific analysis of regional cardiac sympathetic activity in patients undergoing 18F-DOPA PET-CT for evaluation of NET. Significantly higher cardiac 18F-DOPA uptake was observed in women as compared to men. This sex-difference in 18F-DOPA uptake was most pronounced in the apical region of the LV. With advancing age, 18F-DOPA uptake significantly increased in women, in particular in the LV apex, while no age-dependent changes of 18F-DOPA uptake were observed in men.
While physiological uptake of 18F-DOPA in extraneuronal tissue including liver, myocardium and peripheral muscles has been described previously [14, 17], our study is the first to report sex-and age-specific differences in regional myocardial 18F-DOPA uptake in patients without obvious cardiovascular disease. Given its role as precursor of the neurotransmitters dopamine, epinephrine, and norepinephrine, enhanced 18F-DOPA uptake mirrors an increased activity of L-DOPA decarboxylase.[17] The latter is associated with an increased synthesis and turnover of norepinephrine in the adrenergic nerve terminals, thereby signifying an increase in sympathetic outflow. Accordingly, enhanced cardiac L-DOPA decarboxylase activities have been associated with increased conversion of [3H]tyrosine to [3H]catecholamine as well as with a faster metabolic rate of norepinephrine in diabetic rats.[18] Therefore, our observations suggest a sympathetic dominance in the LV apical region which is progressively prominent with aging in women but not in men. While our observations are consistent with earlier studies reporting an increase in systemic sympathetic drive assessed by MSNA or norepinephrine spillover in older subjects, previous MIBG studies have failed to report consistent data on age- and sex-specific cardiac sympathetic activity.[9, 19, 20] In fact, while Sakata et al. observed a gradual decrease of 123I-MIBG uptake alongside an increase in 123I-MIBG washout rate with age in both sexes, Tsuchimochi et al. did not observe any differences in MIBG uptake based on age or sex.[21, 22] Heterogenous cohorts, small sample sizes as well as differences in acquisition protocols might have accounted for these discrepancies. In addition, according to our data, age-dependent changes in LV innervation seem not to be uniform and previous data might be inconsistent due to the assumption that the sympathetic nervous system in the human LV would change homogeneously with age.
While our study clearly demonstrates physiologic changes in regional cardiac sympathetic activity with age, the mechanisms underlying this age-dependent remodeling of cardiac sympathetic outflow remain unclear. Among the postulated mechanisms, age-dependent changes in hormone levels are notable as well as attenuation of parasympathetic activity in postmenopausal women.[23] Indeed, several studies have reported an inhibiting effect of hormone replacement therapy on sympathetic activity in postmenopausal women, while lower testosterone levels have been associated with increased sympathetic excitability in older men with cardiac hypertrophy.[24–26] In addition, an aging-related decline in norepinephrine transporter/uptake-1 activity has been suggested to enhance the delivery of catecholamines to postsynaptic sites.[27] Finally, the observed increase of apical 18F-DOPA uptake in women >55 years of age could be due to a denser sympathetic innervation as a result of an age-dependent remodeling of the female heart. Indeed, a stronger effect of age on LV size and function has been observed in women as compared to men as recent observational studies have reported a higher percentage of small hearts alongside a higher LV ejection fraction in aged females as compared to younger women and age-matched men.[28]
Although cardiac sympathetic function is adversely altered in many disease states, a causal relationship between the observed increase in sympathetic activity in postmenopausal females and their enhanced cardiac vulnerability remains to be established. However, it is known that during an acute coronary syndrome (ACS), women developed a relative greater magnitude of sympathetic activation than men which lasts until its resolution at nine months.[29] The latter is consistent with reports of a worse prognosis in women observed during this time period.[30] In addition, an enhanced vascular transduction of sympathetic activity into hemodynamic parameters due to a decrease in β-adrenergic vasodilatation has been postulated in older women. [31–33] Thus, unopposed α-adrenergic vasoconstriction along with enhanced sympathetic activity might predispose aged females to cardiac susceptibility in high-stress situations. Indeed, cardiac diseases associated with an augmented neural response to mental stress, such as Takotsubo cardiomyopathy, are highly prevalent in postmenopausal females.[3] Notably, the distribution pattern of myocardial 18F-DOPA uptake in women >55 years of age mirrors the distribution of LV dysfunction in Takotsubo cardiomyopathy which is least in the LV base and worst in the cardiac apex, resulting in apical ballooning. Similarly, an epinephrine-dependent switch from beta(2)-adrenoceptor-G(i) to G(s) protein signalling with subsequent negative inotropic effect in stress-induced cardiomyopathy, has been shown to be greatest at the apical myocardium.[34]
In light of the strong association between disease state and sympathetic dysregulation, our data suggest that the quantification of cardiac sympathetic outflow might add prognostic information about cardiac vulnerability in women beyond that provided by traditional risk factors. Indeed, there is accumulating evidence that imaging of cardiac sympathetic activity may help to direct clinical decision making in patients with heart failure, a condition characterized by dysfunction of the sympathetic nervous system.[12] As increased sympathetic tone can be targeted pharmacologically and non-pharmacologically, early identification of patients at risk might offer the possibility to select appropriate preventive strategies and tailor therapeutic approaches. However, despite its FDA approval for heart failure patients, the exact role of cardiac neuronal imaging is still under debate, and, therefore sympathetic imaging modalities such as 123I-Metaiodobenzylguanidine (mIBG) scintigraphy or 11C-mHED PET are still mostly applied as a research method.[12, 35–37] Nevertheless, the limited prognostic value of current diagnostic strategies in women,[38–41] their overall higher cardiovascular mortality, and the increasing prevalence of cardiovascular disease due to the ageing of the population emphasize the need to better identify and understand the substrate that places postmenopausal women at risk. Further research is needed to assess whether sex-differences in cardiac sympathetic activity may account for the higher cardiovascular mortality seen in women and the different outcomes observed in pre- and post-menopausal women. In addition, larger-scale investigations are warranted to delineate whether regional cardiac sympathetic activity adds an incremental prognostic value in predicting cardiovascular risk beyond that provided by traditional functional and neurohormonal markers.
As with any study, certain design limitations are inherent. First, this study is a retrospective analysis from a single center, which limits its generalizability. Second, no follow-up data were available from our study cohort, thus, no conclusions can be made regarding an association between regional cardiac sympathetic activity and the risk of future adverse cardiovascular events. Third, no standardized dose and timing of 18F-DOPA PET currently exists and the 18F-DOPA activity administered varies largely in the literature.[17] However, the 45 min time interval between injection and acquisition as well as the administered dose of 202.8±36.7 MBq in our study lies within in the range of doses and timing reported in recent studies and has been recommended by the 2012 European Association of Nuclear Medicine (EANM) guidelines.[42] Fourth, unspecific uptake of radiolabeled amino acids in inflamed tissue, scar tissue, irradiated or ischemic areas has been reported and cannot be excluded in the present study. However, given that our study subjects did not have abnormalities suspicious of heart disease, it is unlikely that ischemic or inflammatory cardiac processes account for our findings. Finally, although no difference in cardiac 18F-DOPA uptake between 18F-DOPA positive and negative scans were observed, an effect of NET on cardiac DOPA decarboxylase activity cannot be ruled out in our study. Similarly, as myocardial perfusion imaging has not been performed in our study and despite the normalization of myocardial uptake to blood pool 18F-DOPA activity, an effect of myocardial blood flow on tracer kinetics cannot be completely excluded.
Taken together, our study suggests that a sympathetic dominance in the LV apex becomes progressively prominent with aging in women but not in men. Our data point out that understanding the multifactorial nature of cardiac autonomic modulation may provide diagnostic insights for a sex-specific cardiovascular disease management. Such a personalized approach will become ever more important in the upcoming years, given the ageing of the population and the increasing burden of environmental stress and cardiovascular disease. Future studies will have to evaluate whether there is a clinical role for cardiac neuronal imaging in phenotyping patients at risk for future cardiac events.
Supporting information
Normalized myocardial 18F DOPA uptake per patient.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by grants from the Swiss National Science Foundation (SNSF), the Olga Mayenfisch Foundation, Switzerland, the OPO Foundation, Switzerland, the Novartis Foundation, Switzerland, and the Swissheart Foundation. We declare that the authors received research grants from the Swiss National Science Foundation (SNSF), the Olga Mayenfisch Foundation, Switzerland, the OPO Foundation, Switzerland, the Novartis Foundation, Switzerland, the Helmut Horten Foundation, Switzerland, and the Swissheart Foundation (all to Catherine Gebhard). These funding organizations did not play a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript and provided financial support only in the form of salaries (CG, SB, AH, MM) and research materials.
References
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134. [PubMed] [Google Scholar]
- 2.Wilmot KA, O'Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation. 2015;132(11):997–1002. 10.1161/CIRCULATIONAHA.115.015293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373(10):929–38. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 4.Trio O, de Gregorio C, Ando G. Myocardial dysfunction after subarachnoid haemorrhage and tako-tsubo cardiomyopathy: a differential diagnosis? Ther Adv Cardiovasc Dis. 2010;4(2):105–7. 10.1177/1753944709356013 [DOI] [PubMed] [Google Scholar]
- 5.Di Monaco A, Bruno I, Calcagni ML, Nerla R, Lamendola P, Barone L, et al. Cardiac adrenergic nerve function in patients with cardiac syndrome X. J Cardiovasc Med (Hagerstown). 2010;11(3):151–6. [DOI] [PubMed] [Google Scholar]
- 6.Ando G, Trio O, de Gregorio C. Transient left ventricular dysfunction in patients with neurovascular events. Acute Card Care. 2010;12(2):70–4. 10.3109/17482941003732758 [DOI] [PubMed] [Google Scholar]
- 7.Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, et al. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc. 2016;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitoff PR, Gam D, Ivanov J, Al-hesayen A, Azevedo ER, Newton GE, et al. Cardiac-specific sympathetic activation in men and women with and without heart failure. Heart. 2011;97(5):382–7. 10.1136/hrt.2010.199760 [DOI] [PubMed] [Google Scholar]
- 9.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. 1998;275(5 Pt 2):R1600–4. [DOI] [PubMed] [Google Scholar]
- 10.Incognito AV, Doherty CJ, Lee JB, Burns MJ, Millar PJ. Interindividual variability in muscle sympathetic responses to static handgrip in young men: evidence for sympathetic responder types? Am J Physiol Regul Integr Comp Physiol. 2018;314(1):R114–R21. 10.1152/ajpregu.00266.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53(3):571–6. 10.1161/HYPERTENSIONAHA.108.126391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55(20):2212–21. 10.1016/j.jacc.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 13.Ellison DA, Parham DM. Tumors of the Autonomic Nervous System. Pathology Patterns Reviews. 2001;115(suppl_1):S46–S55. [DOI] [PubMed] [Google Scholar]
- 14.Chondrogiannis S, Marzola MC, Rubello D. (1)(8)F-DOPA PET/computed tomography imaging. PET clinics. 2014;9(3):307–21. 10.1016/j.cpet.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. The international journal of cardiovascular imaging. 2002;18(1):539–42. [PubMed] [Google Scholar]
- 16.Takahashi TA, Johnson KM. Menopause. Med Clin North Am. 2015;99(3):521–34. 10.1016/j.mcna.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 17.Chondrogiannis S, Marzola MC, Al-Nahhas A, Venkatanarayana TD, Mazza A, Opocher G, et al. Normal biodistribution pattern and physiologic variants of 18F-DOPA PET imaging. Nucl Med Commun. 2013;34(12):1141–9. 10.1097/MNM.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganguly PK, Dhalla KS, Innes IR, Beamish RE, Dhalla NS. Altered norepinephrine turnover and metabolism in diabetic cardiomyopathy. Circ Res. 1986;59(6):684–93. [DOI] [PubMed] [Google Scholar]
- 19.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45(4):522–5. 10.1161/01.HYP.0000160318.46725.46 [DOI] [PubMed] [Google Scholar]
- 20.Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, et al. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268(1 Pt 2):R278–85. [DOI] [PubMed] [Google Scholar]
- 21.Sakata K, Shirotani M, Yoshida H, Kurata C. Physiological fluctuation of the human left ventricle sympathetic nervous system assessed by iodine-123-MIBG. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1998;39(10):1667–71. [PubMed] [Google Scholar]
- 22.Tsuchimochi S, Tamaki N, Tadamura E, Kawamoto M, Fujita T, Yonekura Y, et al. Age and gender differences in normal myocardial adrenergic neuronal function evaluated by iodine-123-MIBG imaging. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1995;36(6):969–74. [PubMed] [Google Scholar]
- 23.Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiological reviews. 1993;73(4):725–64. 10.1152/physrev.1993.73.4.725 [DOI] [PubMed] [Google Scholar]
- 24.Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103(24):2903–8. [DOI] [PubMed] [Google Scholar]
- 25.Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol. 2003;285(5):H2188–93. 10.1152/ajpheart.00256.2003 [DOI] [PubMed] [Google Scholar]
- 26.Li S, Zhang L, Guo Y, Li X. Relationship of cardiac sympathetic nerve innervation and excitability to cardiac hypertrophy in very elderly male hypertensive patients. High Blood Press Cardiovasc Prev. 2013;20(3):115–21. 10.1007/s40292-013-0018-z [DOI] [PubMed] [Google Scholar]
- 27.Li ST, Holmes C, Kopin IJ, Goldstein DS. Aging-related changes in cardiac sympathetic function in humans, assessed by 6-18F-fluorodopamine PET scanning. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2003;44(10):1599–603. [PubMed] [Google Scholar]
- 28.Gebhard C, Buechel RR, Stahli BE, Gransar H, Achenbach S, Berman DS, et al. Impact of age and sex on left ventricular function determined by coronary computed tomographic angiography: results from the prospective multicentre CONFIRM study. European heart journal cardiovascular Imaging. 2017;18(9):990–1000. [DOI] [PubMed] [Google Scholar]
- 29.Hogarth AJ, Graham LN, Mary DA, Greenwood JP. Gender differences in sympathetic neural activation following uncomplicated acute myocardial infarction. European heart journal. 2009;30(14):1764–70. 10.1093/eurheartj/ehp188 [DOI] [PubMed] [Google Scholar]
- 30.Ubrich R, Barthel P, Haller B, Hnatkova K, Huster KM, Steger A, et al. Sex differences in long-term mortality among acute myocardial infarction patients: Results from the ISAR-RISK and ART studies. PloS one. 2017;12(10):e0186783 10.1371/journal.pone.0186783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briant LJ, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol. 2016;101(2):219–29. 10.1113/EP085368 [DOI] [PubMed] [Google Scholar]
- 32.Harvey RE, Barnes JN, Hart EC, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol. 2017;312(2):H340–H6. 10.1152/ajpheart.00447.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol. 2016;311(6):R1271–R5. 10.1152/ajpregu.00288.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22–9. 10.1038/ncpcardio1066 [DOI] [PubMed] [Google Scholar]
- 35.Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. European journal of nuclear medicine and molecular imaging. 2008;35(3):535–46. 10.1007/s00259-007-0639-3 [DOI] [PubMed] [Google Scholar]
- 36.Ketchum ES, Jacobson AF, Caldwell JH, Senior R, Cerqueira MD, Thomas GS, et al. Selective improvement in Seattle Heart Failure Model risk stratification using iodine-123 meta-iodobenzylguanidine imaging. J Nucl Cardiol. 2012;19(5):1007–16. 10.1007/s12350-012-9603-0 [DOI] [PubMed] [Google Scholar]
- 37.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 38.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130(4):350–79. 10.1161/CIR.0000000000000061 [DOI] [PubMed] [Google Scholar]
- 39.Gianrossi R, Detrano R, Mulvihill D, Lehmann K, Dubach P, Colombo A, et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80(1):87–98. [DOI] [PubMed] [Google Scholar]
- 40.Baldassarre LA, Raman SV, Min JK, Mieres JH, Gulati M, Wenger NK, et al. Noninvasive Imaging to Evaluate Women With Stable Ischemic Heart Disease. JACC Cardiovasc Imaging. 2016;9(4):421–35. 10.1016/j.jcmg.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, et al. Emergence of Nonobstructive Coronary Artery Disease: A Woman's Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66(17):1918–33. 10.1016/j.jacc.2015.08.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taieb D, Timmers HJ, Hindie E, Guillet BA, Neumann HP, Walz MK, et al. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging. 2012;39(12):1977–95. 10.1007/s00259-012-2215-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normalized myocardial 18F DOPA uptake per patient.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.