Abstract
Recognition of facial lentigo maligna (LM) is often difficult, particularly at early stages. Algorithms and multivariate diagnostic models have recently been elaborated on the attempt to improve the diagnostic accuracy. We conducted a cross-sectional and retrospective study to evaluate dermatoscopic criteria aiding in diagnosis of flat pigmented facial lesions (FPFL). We examined 46 FPFL in 42 Caucasian patients and found that 4 of 20 dermatoscopic criteria reached the significance level required for features indicating malignancy namely, hyperpigmented follicular openings, obliterated follicular opening, annular-granular structures, and pigment rhomboids. Concomitant presence of at least 2 or 3 of the 4 mentioned criteria was significantly more frequent in LM than in pigmented actinic keratosis (PAK). However, despite more frequently seen in LM, these features were also displayed in some of the PAK and other FPFL, so we found them not specific for LM. Although dermatoscopy enhances the diagnostic accuracy in evaluating FPFL, histopathology remains the gold standard for correct diagnosis, making evident the need for improvements in early noninvasive diagnosis of LM.
Keywords: dermatoscopy, lentigo maligna, flat pigmented lesions, face
Introduction
Flat pigmented facial lesions (FPFL) on chronic sun-damaged skin include a variety of melanocytic and nonmelanocytic, benign and malignant conditions with a similar clinical appearance presenting as a diagnostic challenge to physicians [1,2]. In many cases, diagnostic uncertainty is not resolved by clinical inspection, leading to biopsy or excision to rule out lentigo maligna (LM) [1].
Recognition of facial melanoma is often difficult, particularly in the early stages. Pigmented lesions of the face do not show the classic dermatoscopic findings characteristically observed elsewhere on the skin. A conventional pigment network is rarely found [2]. Instead, they are dermatoscopically characterized by the presence of a specific feature called a pseudonetwork [2–4]. The well-known “ABCDE rule” cannot be applied to facial locations [5,6]. Differential diagnosis includes solar lentigo (SL), postinflammatory hyperpigmentation (PIH), seborrheic keratosis (SK), pigmented actinic keratosis (PAK), and lichen planus-like keratosis (LPLK) [2]. Dermatoscopy has been demonstrated to be an efficient noninvasive technique for the preoperative assessment, as well as for differential diagnosis of pigmented lesions [5]. For all of these reasons, algorithms and multivariate diagnostic models have recently been elaborated on the attempt to improve the diagnostic accuracy [1,2,5,7].
The aim of the present study was to evaluate dermatoscopic criteria aiding in diagnosis of pigmented skin lesions on the face by blinded evaluation of a consecutive series of dermatoscopic images in order to emphasize their diagnostic value in the differentiation between LM and other FPFL.
Methods
We conducted a cross-sectional and retrospective study of the FPFL in patients attending one author’s office over a 24-month period, from January 2014 to December 2015. We excluded those lesions with equivocal histopathology reports, raised lesions, and lentigo maligna melanoma (LMM) lesions. Concerning this study, the authors refer to the entity as LM when confined to the epidermis (in situ) and as LMM when it invaded the dermis. The gold standard for diagnosis used in this study was the histopathologic report. Clinical and dermatoscopic images of each lesion were documented by a FotoFinder (FotoFinder Systems, Inc, Bad Birnbach, Germany) non-polarized, videodermatoscope and in some cases by a Handyscope (FotoFinder Systems, Inc, Bad Birnbach, Germany) polarized, dermatoscope. In the case of lesions larger than 14 mm in diameter, multiple dermatoscopic images of different areas of the lesion were obtained to provide data for all topographical areas. Immersion fluid, either 70% ethanol hand wash gel, or ultrasound gel, was always used when taking the photographs. All dermatoscopic images were assessed digitally and reviewed by 3 dermatologists (MCS, AC, AMB) before reviewing the histopathologic report.
Dermatoscopic features were positively scored when a consensus of at least 2 of the 3 observers was achieved. Twenty dermatoscopically detectable criteria related to different structures and combinations of colors and structures were analyzed, including scar-like areas, dots, yellow clods, globules, streaks, brown structureless areas, gray structureless areas, milia and comedones [1,2,5,7–10]. Additionally we included other more controversial structures namely:
Rosettes, also called four-dot clods, which are defined as 4 white dots arranged in a square;
Hyperpigmented follicular openings (HFO), considered when fine, irregular, semi-, signet ring or double circles were present;
Obliterated follicular opening (OFO), when obliterated hair follicles were seen;
Pigment rhomboids, interfollicular lines that form a polygon (most commonly a rhomboid);
Moth-eaten borders, defined as concave areas at the edge of the lesion;
Sharp border when there was abrupt cessation of pigmentation;
Scale, evaluated from the dermatoscopic not the clinical image after application of fluid or gel;
Fingerprint-like structures, corresponding to different types of fissures which can be described as ridges, “fat fingers,” or cerebriform pattern;
Annular-granular structures, were considered when granules were found regularly around the follicles;
Red rhomboidal structure, defined as lozenge-shaped vascular pattern occurring in the area separating the hair follicles from each other; and
Increased density of the vascular network, defined as a vascular network of higher density within the lesion than in peripheral skin.
Data Analysis
All features were treated as binary values (present or absent). Statistical methods Chi-squared and Fisher’s exact tests were used to evaluate possible associations. Values of p ≤ 0,05 were considered significant. Data analysis was performed using the statistical software program SPSS 20.0 for Windows (SPSS Inc, Chicago, IL).
Results
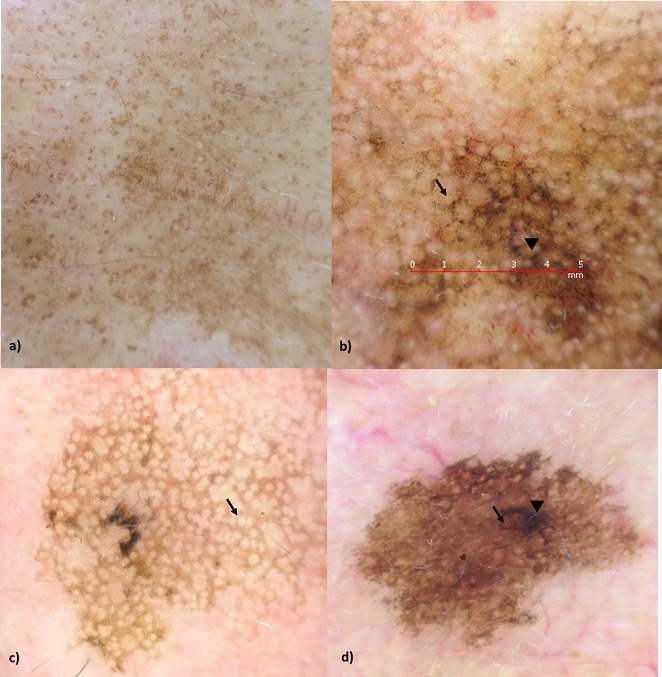
We examined 46 FPFL in 42 Caucasian patients (30 women and 12 men; age range 30–94 years, mean 65,2). LM was diagnosed in 5 (10.9%), PAK in 15 (32.6%), SL in 19 (41.3%), SK in 5 (10,9%), LPLK in 1 (2.2%) and PIH in 1 (2.2%) lesion—all of them histopathologically confirmed. Examples of SL, LM, and PAK are shown in figure 1a–d. Table 1 demonstrates the frequency of detected dermatoscopic features in our series. The most striking pattern in 34 of 46 (73.9%) FPFL was brown structureless areas, followed by HFO (24/46 52.2%). Streaks and milia were not present in any of the lesions observed.
Figure 1.
(a) Solar lentigo with brown structureless areas, (b) lentigo maligna with rhomboids (arrowhead) and annular-granular structures (arrow), (c) pigmented actinic keratosis with rosettes (arrow), (d) pigmented actinic keratosis with hyperpigmented follicular openings (arrow) and obliterated follicular opening (arrowhead). [Copyright: ©2018 Costa-Silva et al.
TABLE 1.
Dermatoscopic Features in a Series of 46 FPFL
| Feature | LM N = 5 | PAK N= 15 | LM + PAK N=20 | Benign Lesions N= 26 | LM + PAK vs Benign Lesions p* | LM vs PAK p* | LM vs FPFL p* | PAK vs FPFL p* |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age (years, median, range) | 66.6 (30–87) | 71 (45–92) | 69.9 (30–92) | 61.6 (30–94) | 0.515 | 0.172 | 0.090 | 0.501 |
|
| ||||||||
| Sex | 0.762 | 0.787 | 0.079 | 0.942 | ||||
| Male | 2 (40%) | 5 (33.3%) | 7 (35%) | 8 (30.8%) | ||||
| Female | 3 (60%) | 10 (66.7%) | 13 (65%) | 18 (69.2%) | ||||
|
| ||||||||
| HFO | 5 (100%) | 9 (60%) | 14 (70%) | 10 (38.5%) | 0.034 | 0.091 | 0.023 | 0.460 |
|
| ||||||||
| Annular-granular structures | 4 (80%) | 8 (53.3%) | 12 (60%) | 6 (23.1%) | 0.011 | 0.292 | 0.047 | 0.170 |
|
| ||||||||
| Pigment rhomboids | 3 (60%) | 1 (6.7%) | 4 (20%) | 2 (7.7%) | 0.219 | 0.010 | 0.001 | 0.372 |
|
| ||||||||
| OFO | 3 (60%) | 1 (6.7%) | 4 (20%) | 4 (15.4%) | 0.682 | 0.010 | 0.008 | 0.182 |
|
| ||||||||
| Combination of 2/4† LM features | 5 (100%) | 6 (40%) | 11 (55%) | 5 (19.2%) | 0.012 | 0.020 | 0.001 | 0.605 |
|
| ||||||||
| Combination of 3/4‡ LM features | 4 (80%) | 1 (6.7%) | 5 (25%) | 2 (7.7%) | 0.105 | 0.001 | 0.001 | 0.261 |
|
| ||||||||
| Scar-like areas | 1 (20%) | 1 (6.7%) | 2 (10%) | 0 | 0.099 | 0.389 | 0.069 | 0.592 |
|
| ||||||||
| Dots | 4 (80%) | 6 (40%) | 10 (50%) | 10 (38.5%) | 0.434 | 0.121 | 0.081 | 0.741 |
|
| ||||||||
| Scale | 0 | 6 (40%) | 6 (30%) | 5 (19.5%) | 0.396 | 0.091 | 0.184 | 0.072 |
|
| ||||||||
| Yellow clods | 1 (20%) | 6 (40%) | 7 (35%) | 4 (15.4%) | 0.122 | 0.417 | 0.828 | 0.075 |
|
| ||||||||
| Rosettes | 0 | 3 (20%) | 3 (15%) | 0 | 0.041 | 0.278 | 0.532 | 0.010 |
|
| ||||||||
| Globules | 2 (40%) | 2 (13.3%) | 5 (25%) | 3 (11.5%) | 0.232 | 0.132 | 0.087 | 0.613 |
|
| ||||||||
| Streaks | 0 | 0 | 0 | 0 | - | - | - | - |
|
| ||||||||
| Brown structureless areas | 4 (80%) | 9 (60%) | 12 (60%) | 22 (84.6%) | 0.159 | 0.292 | 0.743 | 0.075 |
|
| ||||||||
| Fingerprint-like structures | 0 | 1 (6.7%) | 1 (25%) | 7 (26.9%) | 0.052 | 0.554 | 0.277 | 0.182 |
|
| ||||||||
| Sharp border | 2 (40%) | 5 (33.3%) | 7 (35%) | 11 (42.3%) | 0.615 | 0.787 | 0.966 | 0.575 |
|
| ||||||||
| Moth-eaten borders | 0 | 3 (20%) | 3 (15%) | 8 (30.8%) | 0.214 | 0.278 | 0.184 | 0.665 |
|
| ||||||||
| Milia | 0 | 0 | 0 | 0 | - | - | - | - |
|
| ||||||||
| Comedo | 0 | 0 | 0 | 1 (3.8%) | 0.375 | - | 0.724 | 0.482 |
|
| ||||||||
| Red rhomboidal structure | 0 | 1 (6.7%) | 1 (5%) | 0 | 0.249 | 0.554 | 0.724 | 0.146 |
|
| ||||||||
| Increased vascular network | 3 (60%) | 7 (46.7%) | 10 (50%) | 4 (15.4%) | 0.011 | 0.606 | 0.128 | 0.096 |
|
| ||||||||
| Gray structureless areas | 1 (20%) | 3 (20%) | 4 (20%) | 3 (11.5%) | 0.428 | 1 | 0.752 | 0.530 |
HFO- hyperpigmented follicular openings; OFO- obliterated follicular opening; LM – lentigo maligna; PAK – pigmented actinic keratosis; FPFL- flat pigmented facial lesions
Chi-squared and Fisher’s exact tests were used to evaluate possible associations.
Combination of 2 out of the 4 features significantly associated to LM (HFO, OFO, annular-granular structures, and pigment rhomboids).
Combination of 3 out of the 4 features significantly associated to LM (HFO, OFO, annular-granular structures, and pigment rhomboids).
In LM, HFO (5/5, 100%), annular-granular structures (4/5, 80%), brown structureless areas (4/5, 80%), dots (4/5, 80%), OFO (3/5, 60%), pigment rhomboids (3/5, 60%) and increased density of the vascular network (3/5, 60%) were present in most of the lesions. Four features namely, HFO, OFO, annular-granular structures, and pigment rhomboids were significantly associated with LM (p < 0.05). All LMs presented at least 2 out of these 4 features and 4 (80%) LMs had 3 of the 4.
HFO (9/15, 60%), brown structureless areas (9/15, 60%), and annular-granular structures (8/15, 53.3%) were the most common dermatoscopic findings in PAK. Despite being present in only 20% (3/15), rosettes were solely observed in PAK, and this difference was statistically significant when compared to all other FPFL (p < 0.05). No other dermatoscopic criteria were statistically associated with the diagnosis of PAK (p > 0.05).
Concerning premalignant (PAK) and malignant lesions (LM) all together and compared to benign FPFL (namely SL, SK, LPLK and PIH), HFO (70% vs 38.5%, p < 0.05), annular-granular structures (60% vs 23.1%, p < 0,05), rosettes (15% vs 0%, p < 0.05), and increased density of the vascular network (50% vs 15.4%, p < 0.05) were significantly more frequent in the former.
Distinguishing between LM and PAK lesions, pigment rhomboids and OFO were significantly more frequent in the former (60% vs 6.7% and 60% vs 6,7%, respectively, p < 0.05). Concerning features indicating LM namely, HFP, OFO, annular-granular structures, and pigment rhomboids, the concomitant presence of 2 of the 4 mentioned criteria was significantly more frequent in LM than in PAK (100% vs 40%, p < 0,05). Similarly, the concomitant presence of 3 of the 4 mentioned criteria was significantly more frequent in LM than in PAK (80% vs 6.7%, p < 0,05).
Discussion
Caucasian skin chronically exposed to the sun is susceptible to both benign and malignant FPFL [1]. Lentigo maligna is the most common subtype of melanoma on the face with increasing incidence [5]. Despite a frequent delay in diagnosis, its prognosis at the time of diagnosis is globally good [5]. The high frequency of PAK observed in our study mainly reflects its relatively high frequency in the population when compared with LM [9]. Although dermatoscopic characteristics of LM on the face have been described before, knowledge of the significance of dermatoscopic patterns with regard to the differentiation of LM from other FPFL is limited and may be a challenge even for experienced clinicians [1,3,5,7–10].
Schiffner et al [8], found that using a combination of 4 features, asymmetric pigmented follicular openings, dark rhomboidal structures, slate-gray dots, and slate-gray globules resulted in a classification rate of 93% for LM, with a specificity of 96% and a sensitivity of 89%. These criteria differ fundamentally from those of Steiner et al [11], who reported that radial streaming, peripheral black dots, and an irregular prominent pigment network that stops abruptly and thins out at the periphery, were the characteristic features for LM. According to the former findings, a progression model of LM was developed which differentiates 4 steps of the LM invasion of the hair follicles observed by dermatoscopy. Initially HFO appear, then, fine gray dots and globules appear around the follicles, producing the annular-granular pattern. Next, rhomboid pigmented areas are formed in the areas located around the hair follicle openings. Lastly, with progression of the malignant cells within all follicular anatomical structures, the hyperpigmentation coalesces, and OFO emerge [8,12]. Later, Pralong et al [5], confirmed the diagnostic value of the classic Stolz dermatoscopic criteria and described 4 additional features, such as the increased density of the vascular network, red rhomboidal structures, target-like pattern, and darkening at dermatoscopic examination. Akay et al [9], found that 3 dermatoscopic criteria were statistically significant for malignant growth, namely, dark rhomboidal structures, dark streaks, and black blotches. On the other hand, slate-gray globules, annular-granular structure, HFO, and black globules were not statistically significant for either benign or malignant growth. All dermatoscopic findings, except for black blotches, were observed in PAK, leading the authors to conclude that the value of dermatoscopy in differentiating between melanocytic lesions and PAK was limited [3,9]. Recently, Tschandl et al [1], confirmed this assertion, noting that PAK and LM are similar in clinical and dermatoscopic features, sharing the presence of a gray color, although a pattern of pigmented circles is the most common pattern in early LM, while the presence of scales, white circles, and a sharply demarcated border were more in favor of PAK. Tschandl et al [1] saw the presence of gray color in lesions on the face as a strong clue to a malignant process, which is in accordance with the findings of Tiodorovic-Zivkovic et al [13], who postulated that gray color, regardless of the pattern, was the single most sensitive feature for the dermatoscopic recognition of early facial melanoma that can be detected even before the formation of the characteristic LM structures, such as circles or rhomboids. They concluded that its presence should always prompt the clinician to perform a biopsy. Lastly, Carbone et al [2] developed a scoring system to improve the diagnosis of LM with 7 dermatoscopic criteria: HFO, rhomboidal structures, target-like pattern, perifollicular gray color, dark blotches, moth-eaten border, and fingerprint-like structures.
Our analysis is in accordance with the first findings of Schiffner et al and Stolz et al [8,12]. Four of 20 dermatoscopic criteria for analysis of facial pigmented skin lesions reached the significant level required for features indicating malignancy namely, HFO, OFO, annular-granular structures, and pigment rhomboids. The concomitant presence of 2 or 3 of the 4 dermatoscopic criteria enhances the diagnostic value of dermatoscopy in differentiating LM from PAK or from other FPFL. However, despite more frequently seen in LM, these features were also displayed in some of the PAK and other FPFL, so we found them not specific for LM.
Rosettes were solely observed in PAK. However, they can also be found in other non-melanocytic FPFL [1,9]. It must be emphasized that most dermatoscopic images were taken with non-polarized dermatoscope. Because rosettes are mainly visible with polarized light, we might have underestimated the presence of this structure. We found that pigment rhomboids and OFO were significantly more frequent in LM then in PAK but, again, were not specific.
This study reviewed retrospectively a relatively small series of lesions and a larger number of patients and found that facial lesions are important to give more definite results. Also, the lack of a uniform dermatoscopic nomenclature makes it difficult to compare different studies.
Conclusions
Although dermatoscopy improves diagnostic accuracy in evaluating FPFL, it remains a challenge. Histopathology remains the gold standard for correct diagnosis. Improvements in early noninvasive diagnose of LM are needed. Using combinations of dermatoscopic structures may enhance the diagnosis value of dermatoscopy of FPFL.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Tschandl P, Rosendahl C, Kittler H. Dermatoscopy of flat pigmented facial lesions. J Eur Acad Dermatol Venereol. 2015;29(1):120–127. doi: 10.1111/jdv.12483. [DOI] [PubMed] [Google Scholar]
- 2.Carbone A, Ferrari A, Paolino G, et al. Lentigo maligna of the face: A quantitative simple method to identify individual patient risk probability on dermoscopy. Australas J Dermatol. 2017;58(4):286–291. doi: 10.1111/ajd.12595. [DOI] [PubMed] [Google Scholar]
- 3.Pehamberger H, Binder M, Steiner A, Wolff K. In vivo epiluminescence microscopy: improvement of early diagnosis of melanoma. J Invest Dermatol. 1993;100(3 suppl):356S–362S. doi: 10.1038/jid.1993.63. [DOI] [PubMed] [Google Scholar]
- 4.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48(5):679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 5.Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167(2):280–287. doi: 10.1111/j.1365-2133.2012.10932.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197(1):11–17. doi: 10.1159/000017969. [DOI] [PubMed] [Google Scholar]
- 7.Goncharova Y, Attia EAS, Souid K, Vasilenko IV. Dermoscopic features of facial pigmented skin lesions. ISRN Dermatol. 2013;2013:546813. doi: 10.1155/2013/546813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffner R, Schiffner-Rohe J, Vogt T, et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42(1 Pt 1):25–32. doi: 10.1016/S0190-9622(00)90005-7. [DOI] [PubMed] [Google Scholar]
- 9.Akay BN, Kocyigit P, Heper AO, Erdem C. Dermatoscopy of flat pigmented facial lesions: diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br J Dermatol. 2010;163(6):1212–1217. doi: 10.1111/j.1365-2133.2010.10025.x. [DOI] [PubMed] [Google Scholar]
- 10.Stante M, Giorgi V, Stanganelli I, Alfaioli B, Carli P. Dermoscopy for early detection of facial lentigo maligna. Br J Dermatol. 2005;152(2):361–364. doi: 10.1111/j.1365-2133.2004.06328.x. [DOI] [PubMed] [Google Scholar]
- 11.Steiner A, Pehamberger H, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions II. Diagnosis of small pigmented skin lesions and early detection of malignant melanoma. J Am Acad Dermatol. 1987;17(4):584–591. doi: 10.1016/S0190-9622(87)70240-0. [DOI] [PubMed] [Google Scholar]
- 12.Stolz W, Schiffner R, Burgdorf WH. Dermatoscopy for facial pigmented skin lesions. Clin Dermatol. 2002;20(3):276–278. doi: 10.1016/S0738-081X(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 13.Tiodorovic-Zivkovic D, Zalaudek I, Lallas A, Stratigos AJ, Piana S, Argenziano G. The importance of gray color as a dermoscopic clue in facial pigmented lesion evaluation: a case report. Dermatol Pract Concept. 2013;3(4):37–39. doi: 10.5826/dpc.0304a09. [DOI] [PMC free article] [PubMed] [Google Scholar]